Bin Fu
Training-Free Acceleration for Document Parsing Vision-Language Model with Hierarchical Speculative Decoding
Feb 13, 2026Abstract:Document parsing is a fundamental task in multimodal understanding, supporting a wide range of downstream applications such as information extraction and intelligent document analysis. Benefiting from strong semantic modeling and robust generalization, VLM-based end-to-end approaches have emerged as the mainstream paradigm in recent years. However, these models often suffer from substantial inference latency, as they must auto-regressively generate long token sequences when processing long-form documents. In this work, motivated by the extremely long outputs and complex layout structures commonly found in document parsing, we propose a training-free and highly efficient acceleration method. Inspired by speculative decoding, we employ a lightweight document parsing pipeline as a draft model to predict batches of future tokens, while the more accurate VLM verifies these draft predictions in parallel. Moreover, we further exploit the layout-structured nature of documents by partitioning each page into independent regions, enabling parallel decoding of each region using the same draft-verify strategy. The final predictions are then assembled according to the natural reading order. Experimental results demonstrate the effectiveness of our approach: on the general-purpose OmniDocBench, our method provides a 2.42x lossless acceleration for the dots.ocr model, and achieves up to 4.89x acceleration on long-document parsing tasks. We will release our code to facilitate reproducibility and future research.
RGAlign-Rec: Ranking-Guided Alignment for Latent Query Reasoning in Recommendation Systems
Feb 13, 2026Abstract:Proactive intent prediction is a critical capability in modern e-commerce chatbots, enabling "zero-query" recommendations by anticipating user needs from behavioral and contextual signals. However, existing industrial systems face two fundamental challenges: (1) the semantic gap between discrete user features and the semantic intents within the chatbot's Knowledge Base, and (2) the objective misalignment between general-purpose LLM outputs and task-specific ranking utilities. To address these issues, we propose RGAlign-Rec, a closed-loop alignment framework that integrates an LLM-based semantic reasoner with a Query-Enhanced (QE) ranking model. We also introduce Ranking-Guided Alignment (RGA), a multi-stage training paradigm that utilizes downstream ranking signals as feedback to refine the LLM's latent reasoning. Extensive experiments on a large-scale industrial dataset from Shopee demonstrate that RGAlign-Rec achieves a 0.12% gain in GAUC, leading to a significant 3.52% relative reduction in error rate, and a 0.56% improvement in Recall@3. Online A/B testing further validates the cumulative effectiveness of our framework: the Query-Enhanced model (QE-Rec) initially yields a 0.98% improvement in CTR, while the subsequent Ranking-Guided Alignment stage contributes an additional 0.13% gain. These results indicate that ranking-aware alignment effectively synchronizes semantic reasoning with ranking objectives, significantly enhancing both prediction accuracy and service quality in real-world proactive recommendation systems.
Token Pruning for In-Context Generation in Diffusion Transformers
Feb 02, 2026Abstract:In-context generation significantly enhances Diffusion Transformers (DiTs) by enabling controllable image-to-image generation through reference examples. However, the resulting input concatenation drastically increases sequence length, creating a substantial computational bottleneck. Existing token reduction techniques, primarily tailored for text-to-image synthesis, fall short in this paradigm as they apply uniform reduction strategies, overlooking the inherent role asymmetry between reference contexts and target latents across spatial, temporal, and functional dimensions. To bridge this gap, we introduce ToPi, a training-free token pruning framework tailored for in-context generation in DiTs. Specifically, ToPi utilizes offline calibration-driven sensitivity analysis to identify pivotal attention layers, serving as a robust proxy for redundancy estimation. Leveraging these layers, we derive a novel influence metric to quantify the contribution of each context token for selective pruning, coupled with a temporal update strategy that adapts to the evolving diffusion trajectory. Empirical evaluations demonstrate that ToPi can achieve over 30\% speedup in inference while maintaining structural fidelity and visual consistency across complex image generation tasks.
Learning with Geometric Priors in U-Net Variants for Polyp Segmentation
Jan 24, 2026Abstract:Accurate and robust polyp segmentation is essential for early colorectal cancer detection and for computer-aided diagnosis. While convolutional neural network-, Transformer-, and Mamba-based U-Net variants have achieved strong performance, they still struggle to capture geometric and structural cues, especially in low-contrast or cluttered colonoscopy scenes. To address this challenge, we propose a novel Geometric Prior-guided Module (GPM) that injects explicit geometric priors into U-Net-based architectures for polyp segmentation. Specifically, we fine-tune the Visual Geometry Grounded Transformer (VGGT) on a simulated ColonDepth dataset to estimate depth maps of polyp images tailored to the endoscopic domain. These depth maps are then processed by GPM to encode geometric priors into the encoder's feature maps, where they are further refined using spatial and channel attention mechanisms that emphasize both local spatial and global channel information. GPM is plug-and-play and can be seamlessly integrated into diverse U-Net variants. Extensive experiments on five public polyp segmentation datasets demonstrate consistent gains over three strong baselines. Code and the generated depth maps are available at: https://github.com/fvazqu/GPM-PolypSeg
UniPercept: Towards Unified Perceptual-Level Image Understanding across Aesthetics, Quality, Structure, and Texture
Dec 25, 2025



Abstract:Multimodal large language models (MLLMs) have achieved remarkable progress in visual understanding tasks such as visual grounding, segmentation, and captioning. However, their ability to perceive perceptual-level image features remains limited. In this work, we present UniPercept-Bench, a unified framework for perceptual-level image understanding across three key domains: Aesthetics, Quality, Structure and Texture. We establish a hierarchical definition system and construct large-scale datasets to evaluate perceptual-level image understanding. Based on this foundation, we develop a strong baseline UniPercept trained via Domain-Adaptive Pre-Training and Task-Aligned RL, enabling robust generalization across both Visual Rating (VR) and Visual Question Answering (VQA) tasks. UniPercept outperforms existing MLLMs on perceptual-level image understanding and can serve as a plug-and-play reward model for text-to-image generation. This work defines Perceptual-Level Image Understanding in the era of MLLMs and, through the introduction of a comprehensive benchmark together with a strong baseline, provides a solid foundation for advancing perceptual-level multimodal image understanding.
dMLLM-TTS: Self-Verified and Efficient Test-Time Scaling for Diffusion Multi-Modal Large Language Models
Dec 22, 2025



Abstract:Diffusion Multi-modal Large Language Models (dMLLMs) have recently emerged as a novel architecture unifying image generation and understanding. However, developing effective and efficient Test-Time Scaling (TTS) methods to unlock their full generative potential remains an underexplored challenge. To address this, we propose dMLLM-TTS, a novel framework operating on two complementary scaling axes: (1) trajectory exploration scaling to enhance the diversity of generated hypotheses, and (2) iterative refinement scaling for stable generation. Conventional TTS approaches typically perform linear search across these two dimensions, incurring substantial computational costs of O(NT) and requiring an external verifier for best-of-N selection. To overcome these limitations, we propose two innovations. First, we design an efficient hierarchical search algorithm with O(N+T) complexity that adaptively expands and prunes sampling trajectories. Second, we introduce a self-verified feedback mechanism that leverages the dMLLMs' intrinsic image understanding capabilities to assess text-image alignment, eliminating the need for external verifier. Extensive experiments on the GenEval benchmark across three representative dMLLMs (e.g., Lumina-DiMOO, MMaDA, Muddit) show that our framework substantially improves generation quality while achieving up to 6x greater efficiency than linear search. Project page: https://github.com/Alpha-VLLM/Lumina-DiMOO.
A Survey of Scientific Large Language Models: From Data Foundations to Agent Frontiers
Aug 28, 2025



Abstract:Scientific Large Language Models (Sci-LLMs) are transforming how knowledge is represented, integrated, and applied in scientific research, yet their progress is shaped by the complex nature of scientific data. This survey presents a comprehensive, data-centric synthesis that reframes the development of Sci-LLMs as a co-evolution between models and their underlying data substrate. We formulate a unified taxonomy of scientific data and a hierarchical model of scientific knowledge, emphasizing the multimodal, cross-scale, and domain-specific challenges that differentiate scientific corpora from general natural language processing datasets. We systematically review recent Sci-LLMs, from general-purpose foundations to specialized models across diverse scientific disciplines, alongside an extensive analysis of over 270 pre-/post-training datasets, showing why Sci-LLMs pose distinct demands -- heterogeneous, multi-scale, uncertainty-laden corpora that require representations preserving domain invariance and enabling cross-modal reasoning. On evaluation, we examine over 190 benchmark datasets and trace a shift from static exams toward process- and discovery-oriented assessments with advanced evaluation protocols. These data-centric analyses highlight persistent issues in scientific data development and discuss emerging solutions involving semi-automated annotation pipelines and expert validation. Finally, we outline a paradigm shift toward closed-loop systems where autonomous agents based on Sci-LLMs actively experiment, validate, and contribute to a living, evolving knowledge base. Collectively, this work provides a roadmap for building trustworthy, continually evolving artificial intelligence (AI) systems that function as a true partner in accelerating scientific discovery.
InternVL3.5: Advancing Open-Source Multimodal Models in Versatility, Reasoning, and Efficiency
Aug 25, 2025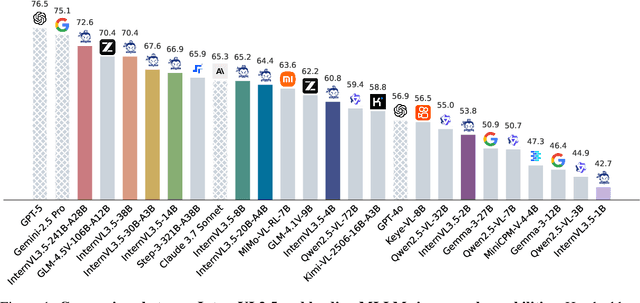
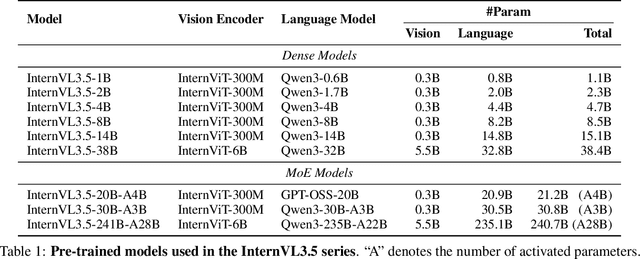
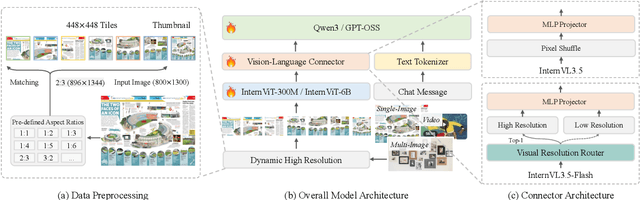
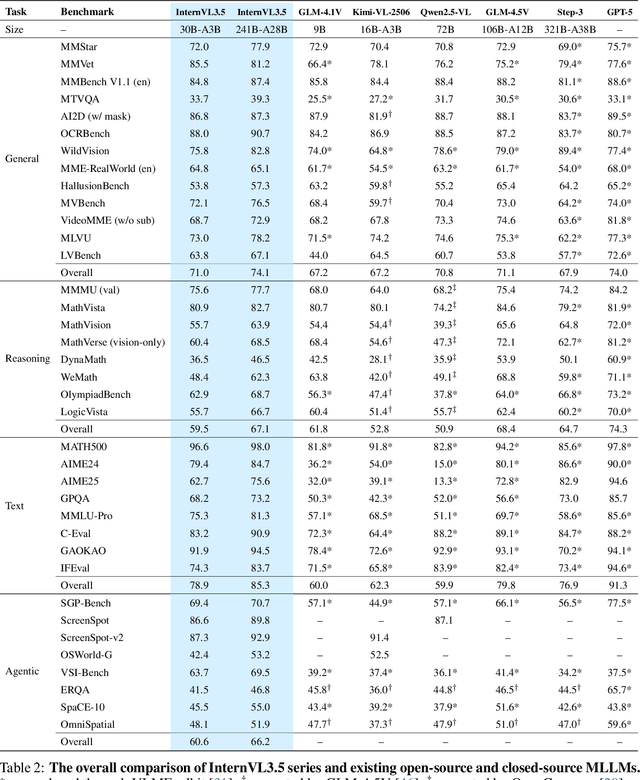
Abstract:We introduce InternVL 3.5, a new family of open-source multimodal models that significantly advances versatility, reasoning capability, and inference efficiency along the InternVL series. A key innovation is the Cascade Reinforcement Learning (Cascade RL) framework, which enhances reasoning through a two-stage process: offline RL for stable convergence and online RL for refined alignment. This coarse-to-fine training strategy leads to substantial improvements on downstream reasoning tasks, e.g., MMMU and MathVista. To optimize efficiency, we propose a Visual Resolution Router (ViR) that dynamically adjusts the resolution of visual tokens without compromising performance. Coupled with ViR, our Decoupled Vision-Language Deployment (DvD) strategy separates the vision encoder and language model across different GPUs, effectively balancing computational load. These contributions collectively enable InternVL3.5 to achieve up to a +16.0\% gain in overall reasoning performance and a 4.05$\times$ inference speedup compared to its predecessor, i.e., InternVL3. In addition, InternVL3.5 supports novel capabilities such as GUI interaction and embodied agency. Notably, our largest model, i.e., InternVL3.5-241B-A28B, attains state-of-the-art results among open-source MLLMs across general multimodal, reasoning, text, and agentic tasks -- narrowing the performance gap with leading commercial models like GPT-5. All models and code are publicly released.
Lumina-mGPT 2.0: Stand-Alone AutoRegressive Image Modeling
Jul 23, 2025
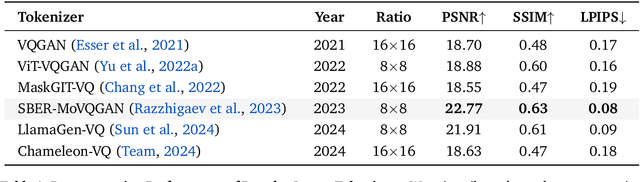
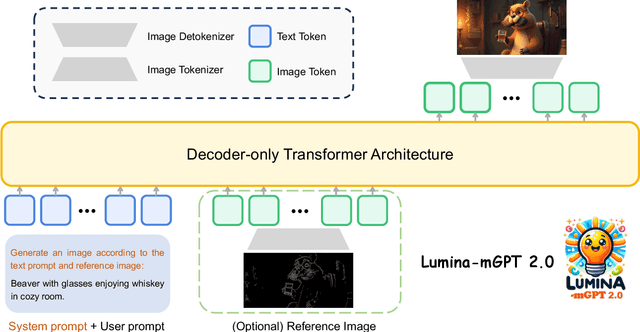
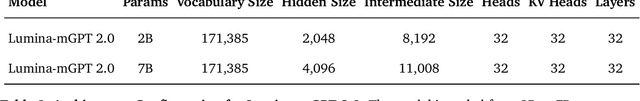
Abstract:We present Lumina-mGPT 2.0, a stand-alone, decoder-only autoregressive model that revisits and revitalizes the autoregressive paradigm for high-quality image generation and beyond. Unlike existing approaches that rely on pretrained components or hybrid architectures, Lumina-mGPT 2.0 is trained entirely from scratch, enabling unrestricted architectural design and licensing freedom. It achieves generation quality on par with state-of-the-art diffusion models such as DALL-E 3 and SANA, while preserving the inherent flexibility and compositionality of autoregressive modeling. Our unified tokenization scheme allows the model to seamlessly handle a wide spectrum of tasks-including subject-driven generation, image editing, controllable synthesis, and dense prediction-within a single generative framework. To further boost usability, we incorporate efficient decoding strategies like inference-time scaling and speculative Jacobi sampling to improve quality and speed, respectively. Extensive evaluations on standard text-to-image benchmarks (e.g., GenEval, DPG) demonstrate that Lumina-mGPT 2.0 not only matches but in some cases surpasses diffusion-based models. Moreover, we confirm its multi-task capabilities on the Graph200K benchmark, with the native Lumina-mGPT 2.0 performing exceptionally well. These results position Lumina-mGPT 2.0 as a strong, flexible foundation model for unified multimodal generation. We have released our training details, code, and models at https://github.com/Alpha-VLLM/Lumina-mGPT-2.0.
Resurrect Mask AutoRegressive Modeling for Efficient and Scalable Image Generation
Jul 17, 2025



Abstract:AutoRegressive (AR) models have made notable progress in image generation, with Masked AutoRegressive (MAR) models gaining attention for their efficient parallel decoding. However, MAR models have traditionally underperformed when compared to standard AR models. This study refines the MAR architecture to improve image generation quality. We begin by evaluating various image tokenizers to identify the most effective one. Subsequently, we introduce an improved Bidirectional LLaMA architecture by replacing causal attention with bidirectional attention and incorporating 2D RoPE, which together form our advanced model, MaskGIL. Scaled from 111M to 1.4B parameters, MaskGIL achieves a FID score of 3.71, matching state-of-the-art AR models in the ImageNet 256x256 benchmark, while requiring only 8 inference steps compared to the 256 steps of AR models. Furthermore, we develop a text-driven MaskGIL model with 775M parameters for generating images from text at various resolutions. Beyond image generation, MaskGIL extends to accelerate AR-based generation and enable real-time speech-to-image conversion. Our codes and models are available at https://github.com/synbol/MaskGIL.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge