Image To Image Translation
Image-to-image translation is the process of converting an image from one domain to another using deep learning techniques.
Papers and Code
FrameDiffuser: G-Buffer-Conditioned Diffusion for Neural Forward Frame Rendering
Dec 18, 2025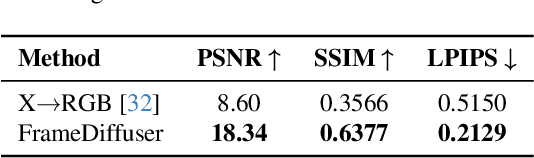
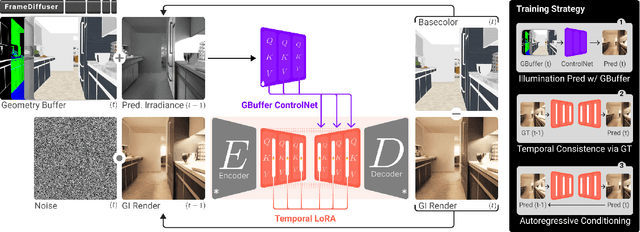
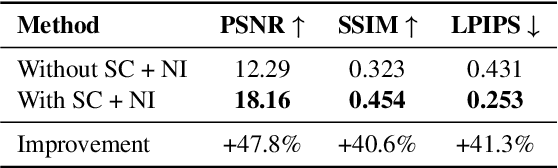
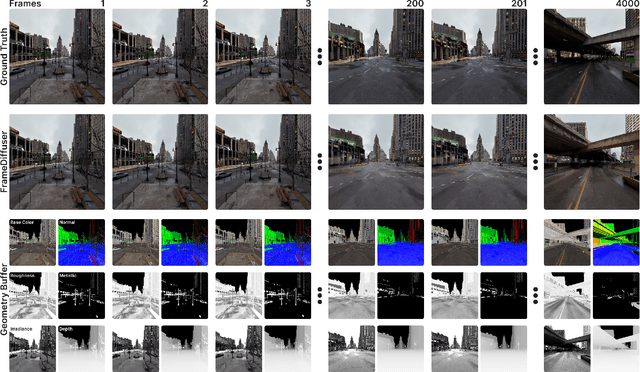
Neural rendering for interactive applications requires translating geometric and material properties (G-buffer) to photorealistic images with realistic lighting on a frame-by-frame basis. While recent diffusion-based approaches show promise for G-buffer-conditioned image synthesis, they face critical limitations: single-image models like RGBX generate frames independently without temporal consistency, while video models like DiffusionRenderer are too computationally expensive for most consumer gaming sets ups and require complete sequences upfront, making them unsuitable for interactive applications where future frames depend on user input. We introduce FrameDiffuser, an autoregressive neural rendering framework that generates temporally consistent, photorealistic frames by conditioning on G-buffer data and the models own previous output. After an initial frame, FrameDiffuser operates purely on incoming G-buffer data, comprising geometry, materials, and surface properties, while using its previously generated frame for temporal guidance, maintaining stable, temporal consistent generation over hundreds to thousands of frames. Our dual-conditioning architecture combines ControlNet for structural guidance with ControlLoRA for temporal coherence. A three-stage training strategy enables stable autoregressive generation. We specialize our model to individual environments, prioritizing consistency and inference speed over broad generalization, demonstrating that environment-specific training achieves superior photorealistic quality with accurate lighting, shadows, and reflections compared to generalized approaches.
MatE: Material Extraction from Single-Image via Geometric Prior
Dec 20, 2025The creation of high-fidelity, physically-based rendering (PBR) materials remains a bottleneck in many graphics pipelines, typically requiring specialized equipment and expert-driven post-processing. To democratize this process, we present MatE, a novel method for generating tileable PBR materials from a single image taken under unconstrained, real-world conditions. Given an image and a user-provided mask, MatE first performs coarse rectification using an estimated depth map as a geometric prior, and then employs a dual-branch diffusion model. Leveraging a learned consistency from rotation-aligned and scale-aligned training data, this model further rectify residual distortions from the coarse result and translate it into a complete set of material maps, including albedo, normal, roughness and height. Our framework achieves invariance to the unknown illumination and perspective of the input image, allowing for the recovery of intrinsic material properties from casual captures. Through comprehensive experiments on both synthetic and real-world data, we demonstrate the efficacy and robustness of our approach, enabling users to create realistic materials from real-world image.
Reframing Music-Driven 2D Dance Pose Generation as Multi-Channel Image Generation
Dec 12, 2025Recent pose-to-video models can translate 2D pose sequences into photorealistic, identity-preserving dance videos, so the key challenge is to generate temporally coherent, rhythm-aligned 2D poses from music, especially under complex, high-variance in-the-wild distributions. We address this by reframing music-to-dance generation as a music-token-conditioned multi-channel image synthesis problem: 2D pose sequences are encoded as one-hot images, compressed by a pretrained image VAE, and modeled with a DiT-style backbone, allowing us to inherit architectural and training advances from modern text-to-image models and better capture high-variance 2D pose distributions. On top of this formulation, we introduce (i) a time-shared temporal indexing scheme that explicitly synchronizes music tokens and pose latents over time and (ii) a reference-pose conditioning strategy that preserves subject-specific body proportions and on-screen scale while enabling long-horizon segment-and-stitch generation. Experiments on a large in-the-wild 2D dance corpus and the calibrated AIST++2D benchmark show consistent improvements over representative music-to-dance methods in pose- and video-space metrics and human preference, and ablations validate the contributions of the representation, temporal indexing, and reference conditioning. See supplementary videos at https://hot-dance.github.io
VASA-3D: Lifelike Audio-Driven Gaussian Head Avatars from a Single Image
Dec 16, 2025We propose VASA-3D, an audio-driven, single-shot 3D head avatar generator. This research tackles two major challenges: capturing the subtle expression details present in real human faces, and reconstructing an intricate 3D head avatar from a single portrait image. To accurately model expression details, VASA-3D leverages the motion latent of VASA-1, a method that yields exceptional realism and vividness in 2D talking heads. A critical element of our work is translating this motion latent to 3D, which is accomplished by devising a 3D head model that is conditioned on the motion latent. Customization of this model to a single image is achieved through an optimization framework that employs numerous video frames of the reference head synthesized from the input image. The optimization takes various training losses robust to artifacts and limited pose coverage in the generated training data. Our experiment shows that VASA-3D produces realistic 3D talking heads that cannot be achieved by prior art, and it supports the online generation of 512x512 free-viewpoint videos at up to 75 FPS, facilitating more immersive engagements with lifelike 3D avatars.
Zero-Shot Textual Explanations via Translating Decision-Critical Features
Dec 08, 2025Textual explanations make image classifier decisions transparent by describing the prediction rationale in natural language. Large vision-language models can generate captions but are designed for general visual understanding, not classifier-specific reasoning. Existing zero-shot explanation methods align global image features with language, producing descriptions of what is visible rather than what drives the prediction. We propose TEXTER, which overcomes this limitation by isolating decision-critical features before alignment. TEXTER identifies the neurons contributing to the prediction and emphasizes the features encoded in those neurons -- i.e., the decision-critical features. It then maps these emphasized features into the CLIP feature space to retrieve textual explanations that reflect the model's reasoning. A sparse autoencoder further improves interpretability, particularly for Transformer architectures. Extensive experiments show that TEXTER generates more faithful and interpretable explanations than existing methods. The code will be publicly released.
MPath: Multimodal Pathology Report Generation from Whole Slide Images
Dec 10, 2025Automated generation of diagnostic pathology reports directly from whole slide images (WSIs) is an emerging direction in computational pathology. Translating high-resolution tissue patterns into clinically coherent text remains difficult due to large morphological variability and the complex structure of pathology narratives. We introduce MPath, a lightweight multimodal framework that conditions a pretrained biomedical language model (BioBART) on WSI-derived visual embeddings through a learned visual-prefix prompting mechanism. Instead of end-to-end vision-language pretraining, MPath leverages foundation-model WSI features (CONCH + Titan) and injects them into BioBART via a compact projection module, keeping the language backbone frozen for stability and data efficiency. MPath was developed and evaluated on the RED 2025 Grand Challenge dataset and ranked 4th in Test Phase 2, despite limited submission opportunities. The results highlight the potential of prompt-based multimodal conditioning as a scalable and interpretable strategy for pathology report generation.
OT-ALD: Aligning Latent Distributions with Optimal Transport for Accelerated Image-to-Image Translation
Nov 14, 2025The Dual Diffusion Implicit Bridge (DDIB) is an emerging image-to-image (I2I) translation method that preserves cycle consistency while achieving strong flexibility. It links two independently trained diffusion models (DMs) in the source and target domains by first adding noise to a source image to obtain a latent code, then denoising it in the target domain to generate the translated image. However, this method faces two key challenges: (1) low translation efficiency, and (2) translation trajectory deviations caused by mismatched latent distributions. To address these issues, we propose a novel I2I translation framework, OT-ALD, grounded in optimal transport (OT) theory, which retains the strengths of DDIB-based approach. Specifically, we compute an OT map from the latent distribution of the source domain to that of the target domain, and use the mapped distribution as the starting point for the reverse diffusion process in the target domain. Our error analysis confirms that OT-ALD eliminates latent distribution mismatches. Moreover, OT-ALD effectively balances faster image translation with improved image quality. Experiments on four translation tasks across three high-resolution datasets show that OT-ALD improves sampling efficiency by 20.29% and reduces the FID score by 2.6 on average compared to the top-performing baseline models.
SAMM2D: Scale-Aware Multi-Modal 2D Dual-Encoder for High-Sensitivity Intracrania Aneurysm Screening
Dec 20, 2025Effective aneurysm detection is essential to avert life-threatening hemorrhages, but it remains challenging due to the subtle morphology of the aneurysm, pronounced class imbalance, and the scarcity of annotated data. We introduce SAMM2D, a dual-encoder framework that achieves an AUC of 0.686 on the RSNA intracranial aneurysm dataset; an improvement of 32% over the clinical baseline. In a comprehensive ablation across six augmentation regimes, we made a striking discovery: any form of data augmentation degraded performance when coupled with a strong pretrained backbone. Our unaugmented baseline model outperformed all augmented variants by 1.75--2.23 percentage points (p < 0.01), overturning the assumption that "more augmentation is always better" in low-data medical settings. We hypothesize that ImageNet-pretrained features already capture robust invariances, rendering additional augmentations both redundant and disruptive to the learned feature manifold. By calibrating the decision threshold, SAMM2D reaches 95% sensitivity, surpassing average radiologist performance, and translates to a projected \$13.9M in savings per 1,000 patients in screening applications. Grad-CAM visualizations confirm that 85% of true positives attend to relevant vascular regions (62% IoU with expert annotations), demonstrating the model's clinically meaningful focus. Our results suggest that future medical imaging workflows could benefit more from strong pretraining than from increasingly complex augmentation pipelines.
FlowBind: Efficient Any-to-Any Generation with Bidirectional Flows
Dec 17, 2025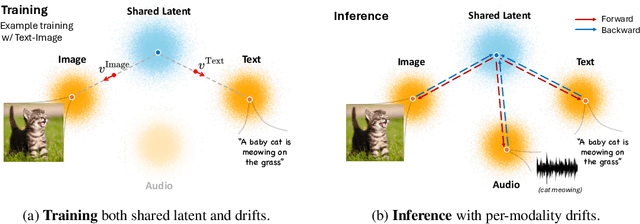
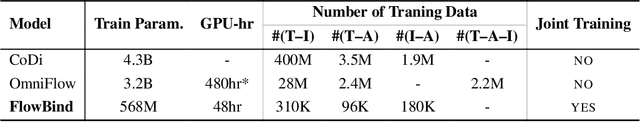
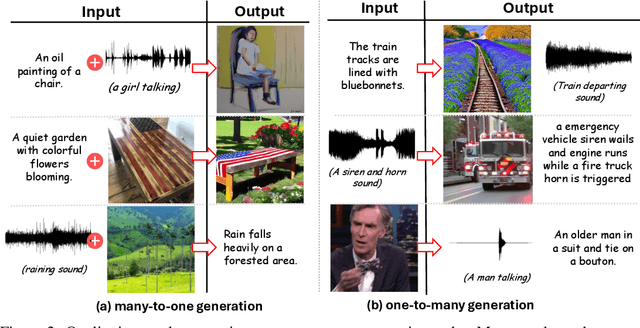
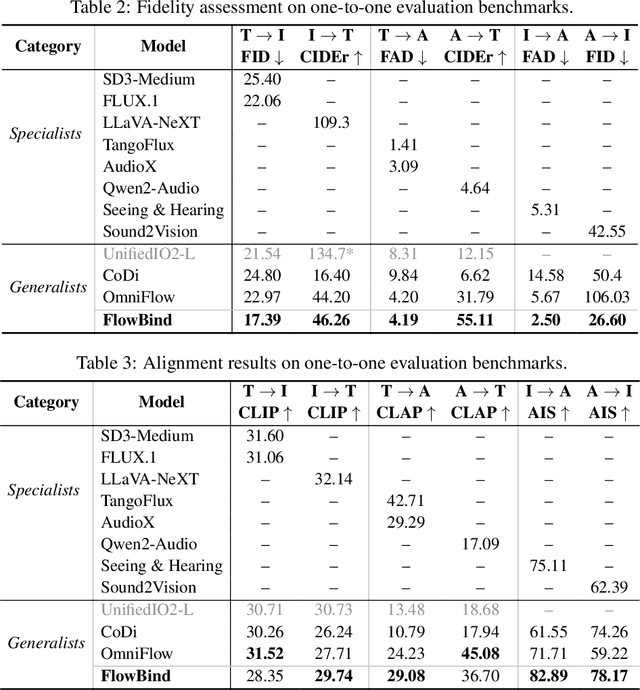
Any-to-any generation seeks to translate between arbitrary subsets of modalities, enabling flexible cross-modal synthesis. Despite recent success, existing flow-based approaches are challenged by their inefficiency, as they require large-scale datasets often with restrictive pairing constraints, incur high computational cost from modeling joint distribution, and rely on complex multi-stage training. We propose FlowBind, an efficient framework for any-to-any generation. Our approach is distinguished by its simplicity: it learns a shared latent space capturing cross-modal information, with modality-specific invertible flows bridging this latent to each modality. Both components are optimized jointly under a single flow-matching objective, and at inference the invertible flows act as encoders and decoders for direct translation across modalities. By factorizing interactions through the shared latent, FlowBind naturally leverages arbitrary subsets of modalities for training, and achieves competitive generation quality while substantially reducing data requirements and computational cost. Experiments on text, image, and audio demonstrate that FlowBind attains comparable quality while requiring up to 6x fewer parameters and training 10x faster than prior methods. The project page with code is available at https://yeonwoo378.github.io/official_flowbind.
VLG-Loc: Vision-Language Global Localization from Labeled Footprint Maps
Dec 18, 2025This paper presents Vision-Language Global Localization (VLG-Loc), a novel global localization method that uses human-readable labeled footprint maps containing only names and areas of distinctive visual landmarks in an environment. While humans naturally localize themselves using such maps, translating this capability to robotic systems remains highly challenging due to the difficulty of establishing correspondences between observed landmarks and those in the map without geometric and appearance details. To address this challenge, VLG-Loc leverages a vision-language model (VLM) to search the robot's multi-directional image observations for the landmarks noted in the map. The method then identifies robot poses within a Monte Carlo localization framework, where the found landmarks are used to evaluate the likelihood of each pose hypothesis. Experimental validation in simulated and real-world retail environments demonstrates superior robustness compared to existing scan-based methods, particularly under environmental changes. Further improvements are achieved through the probabilistic fusion of visual and scan-based localization.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge