Yunxiang Li
Generalized Policy Learning for Smart Grids: FL TRPO Approach
Mar 27, 2024



Abstract:The smart grid domain requires bolstering the capabilities of existing energy management systems; Federated Learning (FL) aligns with this goal as it demonstrates a remarkable ability to train models on heterogeneous datasets while maintaining data privacy, making it suitable for smart grid applications, which often involve disparate data distributions and interdependencies among features that hinder the suitability of linear models. This paper introduces a framework that combines FL with a Trust Region Policy Optimization (FL TRPO) aiming to reduce energy-associated emissions and costs. Our approach reveals latent interconnections and employs personalized encoding methods to capture unique insights, understanding the relationships between features and optimal strategies, allowing our model to generalize to previously unseen data. Experimental results validate the robustness of our approach, affirming its proficiency in effectively learning policy models for smart grid challenges.
FDDM: Unsupervised Medical Image Translation with a Frequency-Decoupled Diffusion Model
Nov 19, 2023



Abstract:Diffusion models have demonstrated significant potential in producing high-quality images for medical image translation to aid disease diagnosis, localization, and treatment. Nevertheless, current diffusion models have limited success in achieving faithful image translations that can accurately preserve the anatomical structures of medical images, especially for unpaired datasets. The preservation of structural and anatomical details is essential to reliable medical diagnosis and treatment planning, as structural mismatches can lead to disease misidentification and treatment errors. In this study, we introduced a frequency-decoupled diffusion model (FDDM), a novel framework that decouples the frequency components of medical images in the Fourier domain during the translation process, to allow structure-preserved high-quality image conversion. FDDM applies an unsupervised frequency conversion module to translate the source medical images into frequency-specific outputs and then uses the frequency-specific information to guide a following diffusion model for final source-to-target image translation. We conducted extensive evaluations of FDDM using a public brain MR-to-CT translation dataset, showing its superior performance against other GAN-, VAE-, and diffusion-based models. Metrics including the Frechet inception distance (FID), the peak signal-to-noise ratio (PSNR), and the structural similarity index measure (SSIM) were assessed. FDDM achieves an FID of 29.88, less than half of the second best. These results demonstrated FDDM's prowess in generating highly-realistic target-domain images while maintaining the faithfulness of translated anatomical structures.
FDNet: Feature Decoupled Segmentation Network for Tooth CBCT Image
Nov 11, 2023Abstract:Precise Tooth Cone Beam Computed Tomography (CBCT) image segmentation is crucial for orthodontic treatment planning. In this paper, we propose FDNet, a Feature Decoupled Segmentation Network, to excel in the face of the variable dental conditions encountered in CBCT scans, such as complex artifacts and indistinct tooth boundaries. The Low-Frequency Wavelet Transform (LF-Wavelet) is employed to enrich the semantic content by emphasizing the global structural integrity of the teeth, while the SAM encoder is leveraged to refine the boundary delineation, thus improving the contrast between adjacent dental structures. By integrating these dual aspects, FDNet adeptly addresses the semantic gap, providing a detailed and accurate segmentation. The framework's effectiveness is validated through rigorous benchmarks, achieving the top Dice and IoU scores of 85.28% and 75.23%, respectively. This innovative decoupling of semantic and boundary features capitalizes on the unique strengths of each element to significantly elevate the quality of segmentation performance.
nnSAM: Plug-and-play Segment Anything Model Improves nnUNet Performance
Oct 02, 2023Abstract:The recent developments of foundation models in computer vision, especially the Segment Anything Model (SAM), allow scalable and domain-agnostic image segmentation to serve as a general-purpose segmentation tool. In parallel, the field of medical image segmentation has benefited significantly from specialized neural networks like the nnUNet, which is trained on domain-specific datasets and can automatically configure the network to tailor to specific segmentation challenges. To combine the advantages of foundation models and domain-specific models, we present nnSAM, which synergistically integrates the SAM model with the nnUNet model to achieve more accurate and robust medical image segmentation. The nnSAM model leverages the powerful and robust feature extraction capabilities of SAM, while harnessing the automatic configuration capabilities of nnUNet to promote dataset-tailored learning. Our comprehensive evaluation of nnSAM model on different sizes of training samples shows that it allows few-shot learning, which is highly relevant for medical image segmentation where high-quality, annotated data can be scarce and costly to obtain. By melding the strengths of both its predecessors, nnSAM positions itself as a potential new benchmark in medical image segmentation, offering a tool that combines broad applicability with specialized efficiency. The code is available at https://github.com/Kent0n-Li/Medical-Image-Segmentation.
Learning from Noisy Labels Generated by Extremely Point Annotations for OCT Fluid Segmentation
Jun 05, 2023Abstract:Automatic segmentation of fluid in OCT (Optical Coherence Tomography) images is beneficial for ophthalmologists to make an accurate diagnosis. Currently, data-driven convolutional neural networks (CNNs) have achieved great success in OCT fluid segmentation. However, obtaining pixel-level masks of OCT images is time-consuming and requires expertise. The popular weakly-supervised strategy is to generate noisy pseudo-labels from weak annotations, but the noise information introduced may mislead the model training. To address this issue, (i) we propose a superpixel-guided method for generating noisy labels from weak point annotations, called Point to Noisy by Superpixel (PNS), which limits the network from over-fitting noise by assigning low confidence to suspiciously noisy label pixels, and (ii) we propose a Two-Stage Mean-Teacher-assisted Confident Learning (2SMTCL) method based on MTCL for multi-category OCT fluid segmentation, which alleviates the uncertainty and computing power consumption introduced by the real-time characterization noise of MTCL. For evaluation, we have constructed a 2D OCT fluid segmentation dataset. Compared with other state-of-art label-denoising methods, comprehensive experimental results demonstrate that the proposed method can achieve excellent performance in OCT fluid segmentation as well as label denoising. Our study provides an efficient, accurate, and practical solution for fluid segmentation of OCT images, which is expected to have a positive impact on the diagnosis and treatment of patients in the field of ophthalmology.
SAMScore: A Semantic Structural Similarity Metric for Image Translation Evaluation
May 24, 2023



Abstract:Image translation has wide applications, such as style transfer and modality conversion, usually aiming to generate images having both high degrees of realism and faithfulness. These problems remain difficult, especially when it is important to preserve semantic structures. Traditional image-level similarity metrics are of limited use, since the semantics of an image are high-level, and not strongly governed by pixel-wise faithfulness to an original image. Towards filling this gap, we introduce SAMScore, a generic semantic structural similarity metric for evaluating the faithfulness of image translation models. SAMScore is based on the recent high-performance Segment Anything Model (SAM), which can perform semantic similarity comparisons with standout accuracy. We applied SAMScore on 19 image translation tasks, and found that it is able to outperform all other competitive metrics on all of the tasks. We envision that SAMScore will prove to be a valuable tool that will help to drive the vibrant field of image translation, by allowing for more precise evaluations of new and evolving translation models. The code is available at https://github.com/Kent0n-Li/SAMScore.
Zero-shot Medical Image Translation via Frequency-Guided Diffusion Models
Apr 05, 2023



Abstract:Recently, the diffusion model has emerged as a superior generative model that can produce high-quality images with excellent realism. There is a growing interest in applying diffusion models to image translation tasks. However, for medical image translation, the existing diffusion models are deficient in accurately retaining structural information since the structure details of source domain images are lost during the forward diffusion process and cannot be fully recovered through learned reverse diffusion, while the integrity of anatomical structures is extremely important in medical images. Training and conditioning diffusion models using paired source and target images with matching anatomy can help. However, such paired data are very difficult and costly to obtain, and may also reduce the robustness of the developed model to out-of-distribution testing data. We propose a frequency-guided diffusion model (FGDM) that employs frequency-domain filters to guide the diffusion model for structure-preserving image translation. Based on its design, FGDM allows zero-shot learning, as it can be trained solely on the data from the target domain, and used directly for source-to-target domain translation without any exposure to the source-domain data during training. We trained FGDM solely on the head-and-neck CT data, and evaluated it on both head-and-neck and lung cone-beam CT (CBCT)-to-CT translation tasks. FGDM outperformed the state-of-the-art methods (GAN-based, VAE-based, and diffusion-based) in all metrics, showing its significant advantages in zero-shot medical image translation.
ChatDoctor: A Medical Chat Model Fine-tuned on LLaMA Model using Medical Domain Knowledge
Apr 01, 2023Abstract:Recent large language models (LLMs) in the general domain, such as ChatGPT, have shown remarkable success in following instructions and producing human-like responses. However, such language models have not been tailored to the medical domain, resulting in poor answer accuracy and inability to give plausible recommendations for medical diagnosis, medications, etc. To address this issue, we collected more than 700 diseases and their corresponding symptoms, required medical tests, and recommended medications, from which we generated 5K doctor-patient conversations. In addition, we obtained 200K real patient-doctor conversations from online Q\&A medical consultation sites. By fine-tuning LLMs using these 205k doctor-patient conversations, the resulting models emerge with great potential to understand patients' needs, provide informed advice, and offer valuable assistance in a variety of medical-related fields. The integration of these advanced language models into healthcare can revolutionize the way healthcare professionals and patients communicate, ultimately improving the overall efficiency and quality of patient care and outcomes. In addition, we made public all the source codes, datasets, and model weights to facilitate the further development of dialogue models in the medical field. The training data, codes, and weights of this project are available at: The training data, codes, and weights of this project are available at: https://github.com/Kent0n-Li/ChatDoctor.
Recurrence-free Survival Prediction under the Guidance of Automatic Gross Tumor Volume Segmentation for Head and Neck Cancers
Sep 22, 2022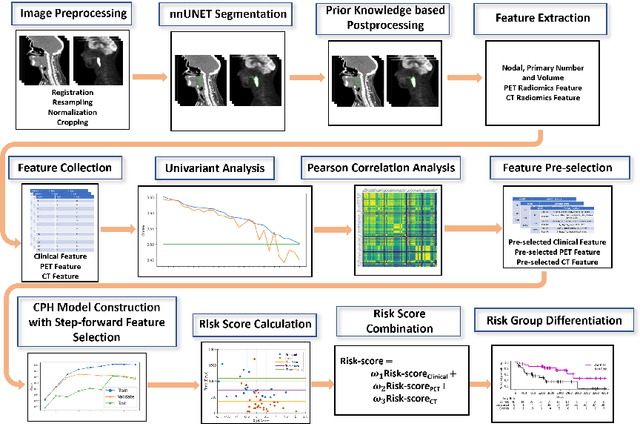
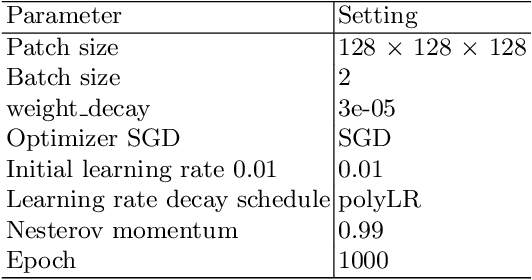
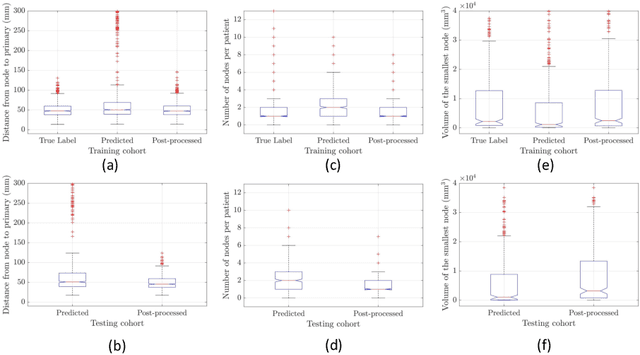
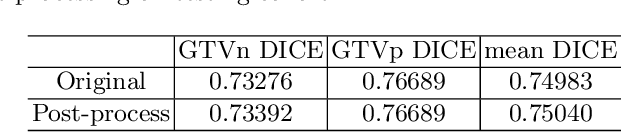
Abstract:For Head and Neck Cancers (HNC) patient management, automatic gross tumor volume (GTV) segmentation and accurate pre-treatment cancer recurrence prediction are of great importance to assist physicians in designing personalized management plans, which have the potential to improve the treatment outcome and quality of life for HNC patients. In this paper, we developed an automated primary tumor (GTVp) and lymph nodes (GTVn) segmentation method based on combined pre-treatment positron emission tomography/computed tomography (PET/CT) scans of HNC patients. We extracted radiomics features from the segmented tumor volume and constructed a multi-modality tumor recurrence-free survival (RFS) prediction model, which fused the prediction results from separate CT radiomics, PET radiomics, and clinical models. We performed 5-fold cross-validation to train and evaluate our methods on the MICCAI 2022 HEad and neCK TumOR segmentation and outcome prediction challenge (HECKTOR) dataset. The ensemble prediction results on the testing cohort achieved Dice scores of 0.77 and 0.73 for GTVp and GTVn segmentation, respectively, and a C-index value of 0.67 for RFS prediction. The code is publicly available (https://github.com/wangkaiwan/HECKTOR-2022-AIRT). Our team's name is AIRT.
LViT: Language meets Vision Transformer in Medical Image Segmentation
Jun 29, 2022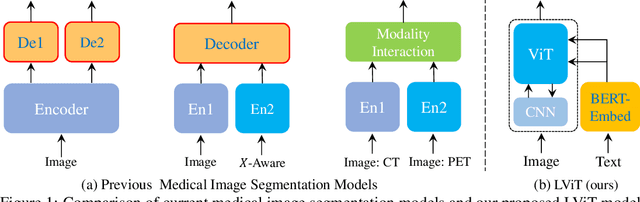
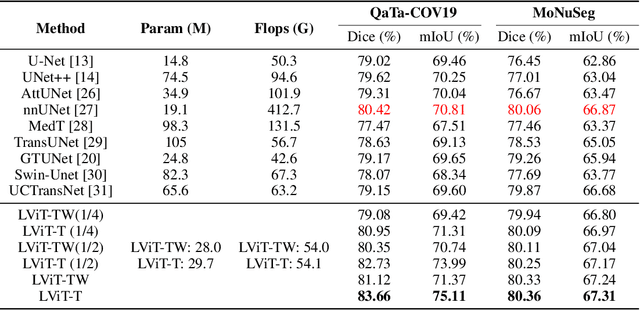
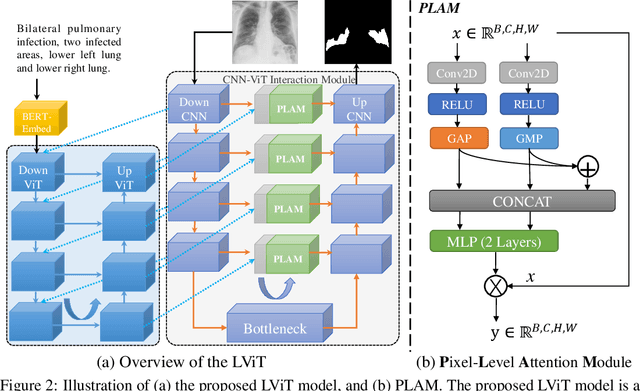
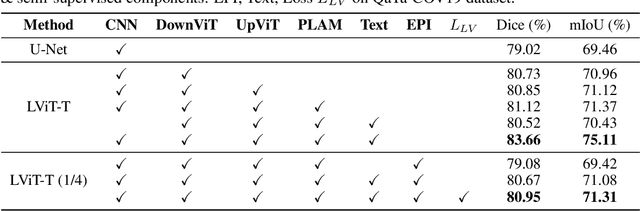
Abstract:Deep learning has been widely used in medical image segmentation and other aspects. However, the performance of existing medical image segmentation models has been limited by the challenge of obtaining sufficient number of high-quality data with the high cost of data annotation. To overcome the limitation, we propose a new vision-language medical image segmentation model LViT (Language meets Vision Transformer). In our model, medical text annotation is introduced to compensate for the quality deficiency in image data. In addition, the text information can guide the generation of pseudo labels to a certain extent and further guarantee the quality of pseudo labels in semi-supervised learning. We also propose the Exponential Pseudo label Iteration mechanism (EPI) to help extend the semi-supervised version of LViT and the Pixel-Level Attention Module (PLAM) to preserve local features of images. In our model, LV (Language-Vision) loss is designed to supervise the training of unlabeled images using text information directly. To validate the performance of LViT, we construct multimodal medical segmentation datasets (image + text) containing pathological images, X-rays,etc. Experimental results show that our proposed LViT has better segmentation performance in both fully and semi-supervised conditions. Code and datasets are available at https://github.com/HUANGLIZI/LViT.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge