Hrvoje Bogunović
on behalf of the PINNACLE consortium
Self-supervised learning via inter-modal reconstruction and feature projection networks for label-efficient 3D-to-2D segmentation
Jul 13, 2023Abstract:Deep learning has become a valuable tool for the automation of certain medical image segmentation tasks, significantly relieving the workload of medical specialists. Some of these tasks require segmentation to be performed on a subset of the input dimensions, the most common case being 3D-to-2D. However, the performance of existing methods is strongly conditioned by the amount of labeled data available, as there is currently no data efficient method, e.g. transfer learning, that has been validated on these tasks. In this work, we propose a novel convolutional neural network (CNN) and self-supervised learning (SSL) method for label-efficient 3D-to-2D segmentation. The CNN is composed of a 3D encoder and a 2D decoder connected by novel 3D-to-2D blocks. The SSL method consists of reconstructing image pairs of modalities with different dimensionality. The approach has been validated in two tasks with clinical relevance: the en-face segmentation of geographic atrophy and reticular pseudodrusen in optical coherence tomography. Results on different datasets demonstrate that the proposed CNN significantly improves the state of the art in scenarios with limited labeled data by up to 8% in Dice score. Moreover, the proposed SSL method allows further improvement of this performance by up to 23%, and we show that the SSL is beneficial regardless of the network architecture.
PALM: Open Fundus Photograph Dataset with Pathologic Myopia Recognition and Anatomical Structure Annotation
May 13, 2023Abstract:Pathologic myopia (PM) is a common blinding retinal degeneration suffered by highly myopic population. Early screening of this condition can reduce the damage caused by the associated fundus lesions and therefore prevent vision loss. Automated diagnostic tools based on artificial intelligence methods can benefit this process by aiding clinicians to identify disease signs or to screen mass populations using color fundus photographs as inputs. This paper provides insights about PALM, our open fundus imaging dataset for pathological myopia recognition and anatomical structure annotation. Our databases comprises 1200 images with associated labels for the pathologic myopia category and manual annotations of the optic disc, the position of the fovea and delineations of lesions such as patchy retinal atrophy (including peripapillary atrophy) and retinal detachment. In addition, this paper elaborates on other details such as the labeling process used to construct the database, the quality and characteristics of the samples and provides other relevant usage notes.
Morph-SSL: Self-Supervision with Longitudinal Morphing to Predict AMD Progression from OCT
Apr 17, 2023



Abstract:The lack of reliable biomarkers makes predicting the conversion from intermediate to neovascular age-related macular degeneration (iAMD, nAMD) a challenging task. We develop a Deep Learning (DL) model to predict the future risk of conversion of an eye from iAMD to nAMD from its current OCT scan. Although eye clinics generate vast amounts of longitudinal OCT scans to monitor AMD progression, only a small subset can be manually labeled for supervised DL. To address this issue, we propose Morph-SSL, a novel Self-supervised Learning (SSL) method for longitudinal data. It uses pairs of unlabelled OCT scans from different visits and involves morphing the scan from the previous visit to the next. The Decoder predicts the transformation for morphing and ensures a smooth feature manifold that can generate intermediate scans between visits through linear interpolation. Next, the Morph-SSL trained features are input to a Classifier which is trained in a supervised manner to model the cumulative probability distribution of the time to conversion with a sigmoidal function. Morph-SSL was trained on unlabelled scans of 399 eyes (3570 visits). The Classifier was evaluated with a five-fold cross-validation on 2418 scans from 343 eyes with clinical labels of the conversion date. The Morph-SSL features achieved an AUC of 0.766 in predicting the conversion to nAMD within the next 6 months, outperforming the same network when trained end-to-end from scratch or pre-trained with popular SSL methods. Automated prediction of the future risk of nAMD onset can enable timely treatment and individualized AMD management.
AIROGS: Artificial Intelligence for RObust Glaucoma Screening Challenge
Feb 10, 2023



Abstract:The early detection of glaucoma is essential in preventing visual impairment. Artificial intelligence (AI) can be used to analyze color fundus photographs (CFPs) in a cost-effective manner, making glaucoma screening more accessible. While AI models for glaucoma screening from CFPs have shown promising results in laboratory settings, their performance decreases significantly in real-world scenarios due to the presence of out-of-distribution and low-quality images. To address this issue, we propose the Artificial Intelligence for Robust Glaucoma Screening (AIROGS) challenge. This challenge includes a large dataset of around 113,000 images from about 60,000 patients and 500 different screening centers, and encourages the development of algorithms that are robust to ungradable and unexpected input data. We evaluated solutions from 14 teams in this paper, and found that the best teams performed similarly to a set of 20 expert ophthalmologists and optometrists. The highest-scoring team achieved an area under the receiver operating characteristic curve of 0.99 (95% CI: 0.98-0.99) for detecting ungradable images on-the-fly. Additionally, many of the algorithms showed robust performance when tested on three other publicly available datasets. These results demonstrate the feasibility of robust AI-enabled glaucoma screening.
Clustering disease trajectories in contrastive feature space for biomarker discovery in age-related macular degeneration
Jan 11, 2023Abstract:Age-related macular degeneration (AMD) is the leading cause of blindness in the elderly. Despite this, the exact dynamics of disease progression are poorly understood. There is a clear need for imaging biomarkers in retinal optical coherence tomography (OCT) that aid the diagnosis, prognosis and management of AMD. However, current grading systems, which coarsely group disease stage into broad categories describing early and intermediate AMD, have very limited prognostic value for the conversion to late AMD. In this paper, we are the first to analyse disease progression as clustered trajectories in a self-supervised feature space. Our method first pretrains an encoder with contrastive learning to project images from longitudinal time series to points in feature space. This enables the creation of disease trajectories, which are then denoised, partitioned and grouped into clusters. These clusters, found in two datasets containing time series of 7,912 patients imaged over eight years, were correlated with known OCT biomarkers. This reinforced efforts by four expert ophthalmologists to investigate clusters, during a clinical comparison and interpretation task, as candidates for time-dependent biomarkers that describe progression of AMD.
Metadata-enhanced contrastive learning from retinal optical coherence tomography images
Aug 04, 2022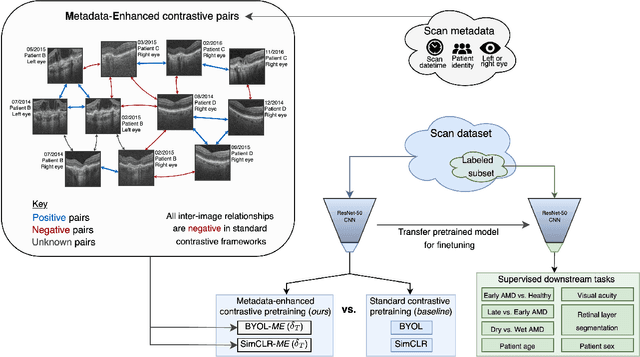
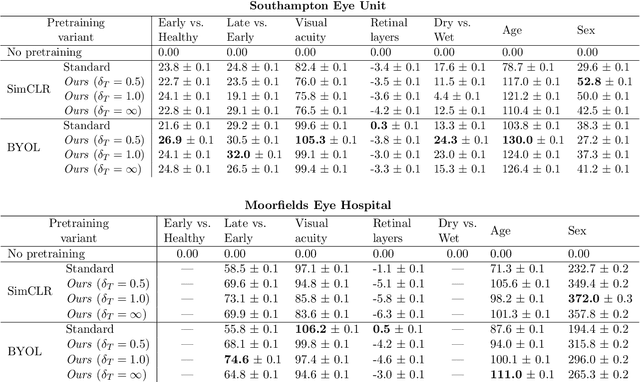
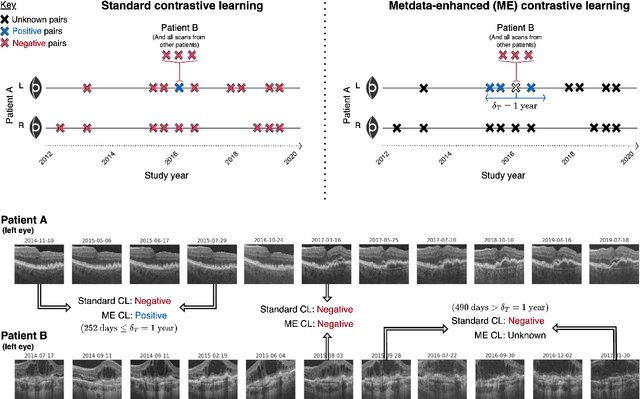
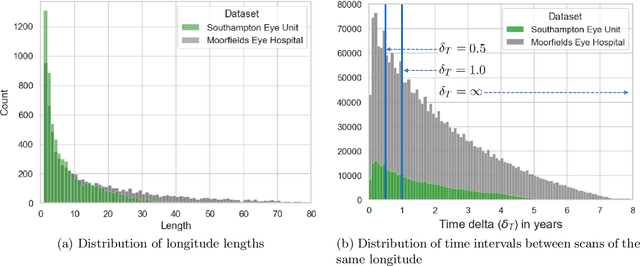
Abstract:Supervised deep learning algorithms hold great potential to automate screening, monitoring and grading of medical images. However, training performant models has typically required vast quantities of labelled data, which is scarcely available in the medical domain. Self-supervised contrastive frameworks relax this dependency by first learning from unlabelled images. In this work we show that pretraining with two contrastive methods, SimCLR and BYOL, improves the utility of deep learning with regard to the clinical assessment of age-related macular degeneration (AMD). In experiments using two large clinical datasets containing 170,427 optical coherence tomography (OCT) images of 7,912 patients, we evaluate benefits attributed to pretraining across seven downstream tasks ranging from AMD stage and type classification to prediction of functional endpoints to segmentation of retinal layers, finding performance significantly increased in six out of seven tasks with fewer labels. However, standard contrastive frameworks have two known weaknesses that are detrimental to pretraining in the medical domain. Several of the image transformations used to create positive contrastive pairs are not applicable to greyscale medical scans. Furthermore, medical images often depict the same anatomical region and disease severity, resulting in numerous misleading negative pairs. To address these issues we develop a novel metadata-enhanced approach that exploits the rich set of inherently available patient information. To this end we employ records for patient identity, eye position (i.e. left or right) and time series data to indicate the typically unknowable set of inter-image contrastive relationships. By leveraging this often neglected information our metadata-enhanced contrastive pretraining leads to further benefits and outperforms conventional contrastive methods in five out of seven downstream tasks.
TINC: Temporally Informed Non-Contrastive Learning for Disease Progression Modeling in Retinal OCT Volumes
Jun 30, 2022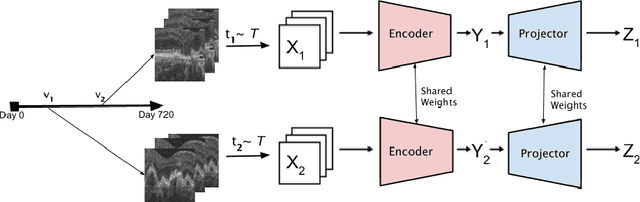
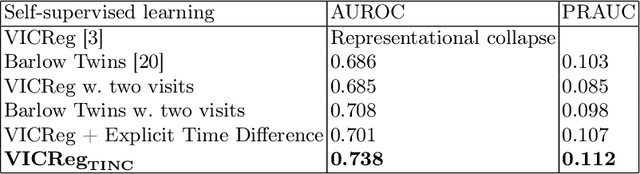
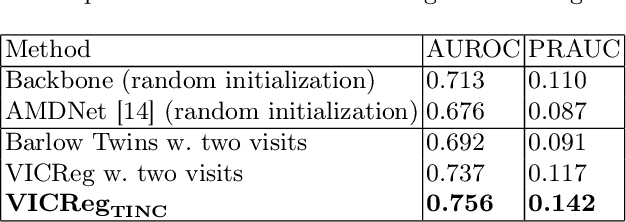
Abstract:Recent contrastive learning methods achieved state-of-the-art in low label regimes. However, the training requires large batch sizes and heavy augmentations to create multiple views of an image. With non-contrastive methods, the negatives are implicitly incorporated in the loss, allowing different images and modalities as pairs. Although the meta-information (i.e., age, sex) in medical imaging is abundant, the annotations are noisy and prone to class imbalance. In this work, we exploited already existing temporal information (different visits from a patient) in a longitudinal optical coherence tomography (OCT) dataset using temporally informed non-contrastive loss (TINC) without increasing complexity and need for negative pairs. Moreover, our novel pair-forming scheme can avoid heavy augmentations and implicitly incorporates the temporal information in the pairs. Finally, these representations learned from the pretraining are more successful in predicting disease progression where the temporal information is crucial for the downstream task. More specifically, our model outperforms existing models in predicting the risk of conversion within a time frame from intermediate age-related macular degeneration (AMD) to the late wet-AMD stage.
REFUGE2 Challenge: Treasure for Multi-Domain Learning in Glaucoma Assessment
Feb 24, 2022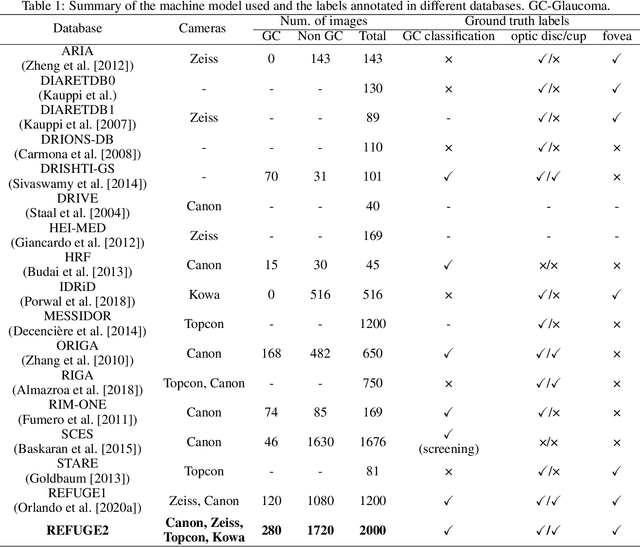
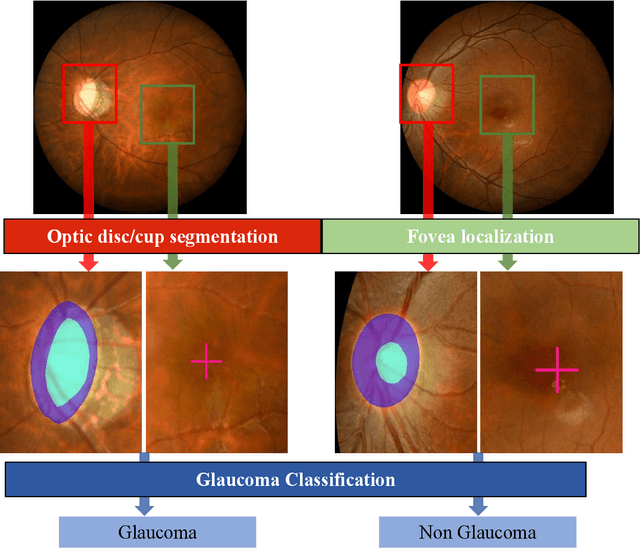
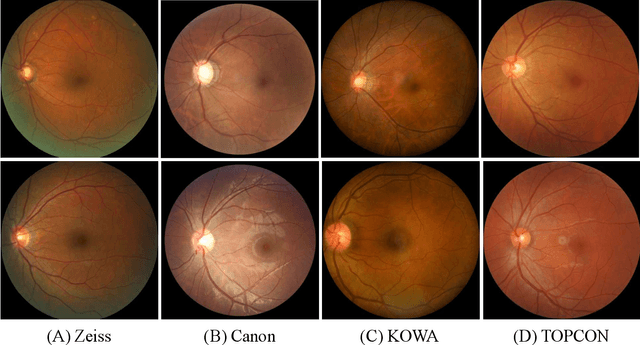
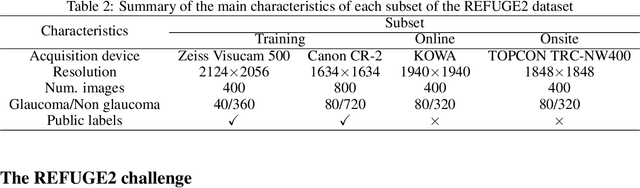
Abstract:Glaucoma is the second leading cause of blindness and is the leading cause of irreversible blindness disease in the world. Early screening for glaucoma in the population is significant. Color fundus photography is the most cost effective imaging modality to screen for ocular diseases. Deep learning network is often used in color fundus image analysis due to its powful feature extraction capability. However, the model training of deep learning method needs a large amount of data, and the distribution of data should be abundant for the robustness of model performance. To promote the research of deep learning in color fundus photography and help researchers further explore the clinical application signification of AI technology, we held a REFUGE2 challenge. This challenge released 2,000 color fundus images of four models, including Zeiss, Canon, Kowa and Topcon, which can validate the stabilization and generalization of algorithms on multi-domain. Moreover, three sub-tasks were designed in the challenge, including glaucoma classification, cup/optic disc segmentation, and macular fovea localization. These sub-tasks technically cover the three main problems of computer vision and clinicly cover the main researchs of glaucoma diagnosis. Over 1,300 international competitors joined the REFUGE2 challenge, 134 teams submitted more than 3,000 valid preliminary results, and 22 teams reached the final. This article summarizes the methods of some of the finalists and analyzes their results. In particular, we observed that the teams using domain adaptation strategies had high and robust performance on the dataset with multi-domain. This indicates that UDA and other multi-domain related researches will be the trend of deep learning field in the future, and our REFUGE2 datasets will play an important role in these researches.
ADAM Challenge: Detecting Age-related Macular Degeneration from Fundus Images
Feb 18, 2022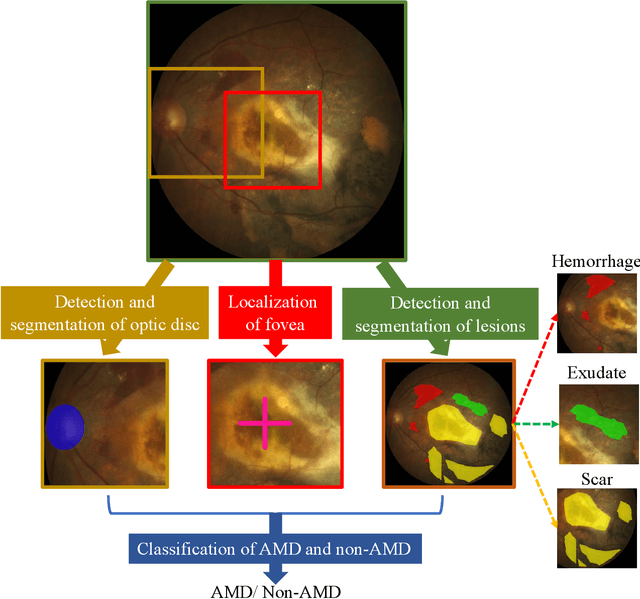
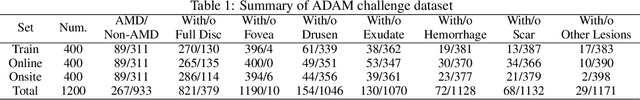
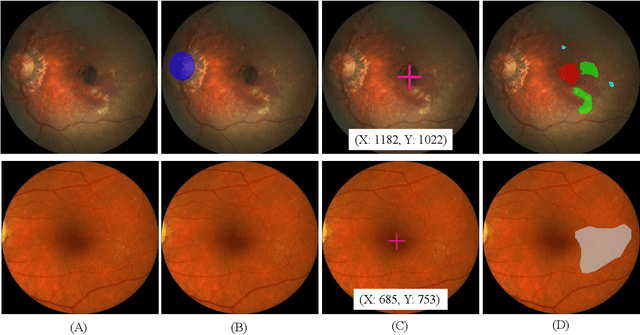
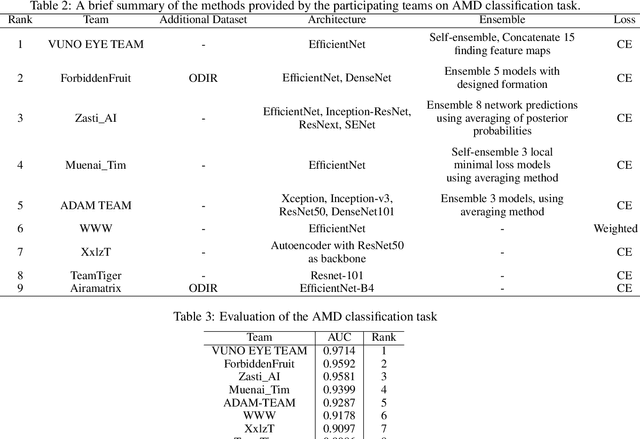
Abstract:Age-related macular degeneration (AMD) is the leading cause of visual impairment among elderly in the world. Early detection of AMD is of great importance as the vision loss caused by AMD is irreversible and permanent. Color fundus photography is the most cost-effective imaging modality to screen for retinal disorders. \textcolor{red}{Recently, some algorithms based on deep learning had been developed for fundus image analysis and automatic AMD detection. However, a comprehensive annotated dataset and a standard evaluation benchmark are still missing.} To deal with this issue, we set up the Automatic Detection challenge on Age-related Macular degeneration (ADAM) for the first time, held as a satellite event of the ISBI 2020 conference. The ADAM challenge consisted of four tasks which cover the main topics in detecting AMD from fundus images, including classification of AMD, detection and segmentation of optic disc, localization of fovea, and detection and segmentation of lesions. The ADAM challenge has released a comprehensive dataset of 1200 fundus images with the category labels of AMD, the pixel-wise segmentation masks of the full optic disc and lesions (drusen, exudate, hemorrhage, scar, and other), as well as the location coordinates of the macular fovea. A uniform evaluation framework has been built to make a fair comparison of different models. During the ADAM challenge, 610 results were submitted for online evaluation, and finally, 11 teams participated in the onsite challenge. This paper introduces the challenge, dataset, and evaluation methods, as well as summarizes the methods and analyzes the results of the participating teams of each task. In particular, we observed that ensembling strategy and clinical prior knowledge can better improve the performances of the deep learning models.
U-Net with spatial pyramid pooling for drusen segmentation in optical coherence tomography
Dec 11, 2019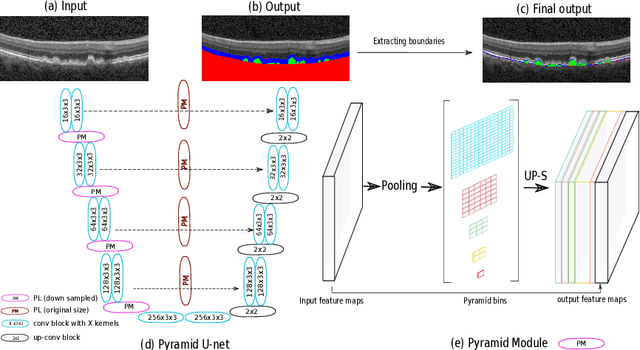

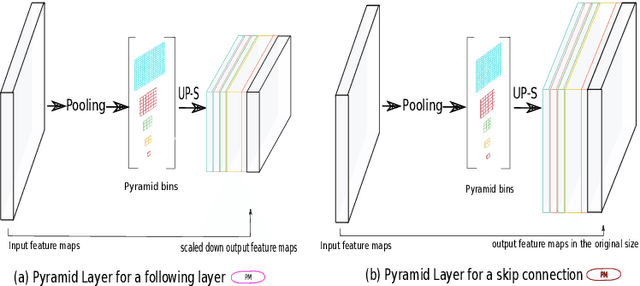
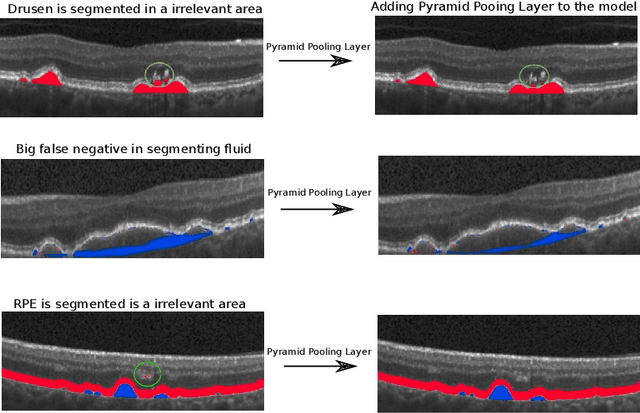
Abstract:The presence of drusen is the main hallmark of early/intermediate age-related macular degeneration (AMD). Therefore, automated drusen segmentation is an important step in image-guided management of AMD. There are two common approaches to drusen segmentation. In the first, the drusen are segmented directly as a binary classification task. In the second approach, the surrounding retinal layers (outer boundary retinal pigment epithelium (OBRPE) and Bruch's membrane (BM)) are segmented and the remaining space between these two layers is extracted as drusen. In this work, we extend the standard U-Net architecture with spatial pyramid pooling components to introduce global feature context. We apply the model to the task of segmenting drusen together with BM and OBRPE. The proposed network was trained and evaluated on a longitudinal OCT dataset of 425 scans from 38 patients with early/intermediate AMD. This preliminary study showed that the proposed network consistently outperformed the standard U-net model.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge