Metal Artifact Reduction
Metal-artifact reduction is the process of reducing image artifacts caused by metal objects in medical images.
Papers and Code
MASC: Metal-Aware Sampling and Correction via Reinforcement Learning for Accelerated MRI
Jan 30, 2026Metal implants in MRI cause severe artifacts that degrade image quality and hinder clinical diagnosis. Traditional approaches address metal artifact reduction (MAR) and accelerated MRI acquisition as separate problems. We propose MASC, a unified reinforcement learning framework that jointly optimizes metal-aware k-space sampling and artifact correction for accelerated MRI. To enable supervised training, we construct a paired MRI dataset using physics-based simulation, generating k-space data and reconstructions for phantoms with and without metal implants. This paired dataset provides simulated 3D MRI scans with and without metal implants, where each metal-corrupted sample has an exactly matched clean reference, enabling direct supervision for both artifact reduction and acquisition policy learning. We formulate active MRI acquisition as a sequential decision-making problem, where an artifact-aware Proximal Policy Optimization (PPO) agent learns to select k-space phase-encoding lines under a limited acquisition budget. The agent operates on undersampled reconstructions processed through a U-Net-based MAR network, learning patterns that maximize reconstruction quality. We further propose an end-to-end training scheme where the acquisition policy learns to select k-space lines that best support artifact removal while the MAR network simultaneously adapts to the resulting undersampling patterns. Experiments demonstrate that MASC's learned policies outperform conventional sampling strategies, and end-to-end training improves performance compared to using a frozen pre-trained MAR network, validating the benefit of joint optimization. Cross-dataset experiments on FastMRI with physics-based artifact simulation further confirm generalization to realistic clinical MRI data. The code and models of MASC have been made publicly available: https://github.com/hrlblab/masc
Physically-Grounded Manifold Projection Model for Generalizable Metal Artifact Reduction in Dental CBCT
Jan 01, 2026Metal artifacts in Dental CBCT severely obscure anatomical structures, hindering diagnosis. Current deep learning for Metal Artifact Reduction (MAR) faces limitations: supervised methods suffer from spectral blurring due to "regression-to-the-mean", while unsupervised ones risk structural hallucinations. Denoising Diffusion Models (DDPMs) offer realism but rely on slow, stochastic iterative sampling, unsuitable for clinical use. To resolve this, we propose the Physically-Grounded Manifold Projection (PGMP) framework. First, our Anatomically-Adaptive Physics Simulation (AAPS) pipeline synthesizes high-fidelity training pairs via Monte Carlo spectral modeling and patient-specific digital twins, bridging the synthetic-to-real gap. Second, our DMP-Former adapts the Direct x-Prediction paradigm, reformulating restoration as a deterministic manifold projection to recover clean anatomy in a single forward pass, eliminating stochastic sampling. Finally, a Semantic-Structural Alignment (SSA) module anchors the solution using priors from medical foundation models (MedDINOv3), ensuring clinical plausibility. Experiments on synthetic and multi-center clinical datasets show PGMP outperforms state-of-the-art methods on unseen anatomy, setting new benchmarks in efficiency and diagnostic reliability. Code and data: https://github.com/ricoleehduu/PGMP.
Physically-Grounded Manifold Projection with Foundation Priors for Metal Artifact Reduction in Dental CBCT
Dec 30, 2025Metal artifacts in Dental CBCT severely obscure anatomical structures, hindering diagnosis. Current deep learning for Metal Artifact Reduction (MAR) faces limitations: supervised methods suffer from spectral blurring due to "regression-to-the-mean", while unsupervised ones risk structural hallucinations. Denoising Diffusion Models (DDPMs) offer realism but rely on slow, stochastic iterative sampling, unsuitable for clinical use. To resolve this, we propose the Physically-Grounded Manifold Projection (PGMP) framework. First, our Anatomically-Adaptive Physics Simulation (AAPS) pipeline synthesizes high-fidelity training pairs via Monte Carlo spectral modeling and patient-specific digital twins, bridging the synthetic-to-real gap. Second, our DMP-Former adapts the Direct x-Prediction paradigm, reformulating restoration as a deterministic manifold projection to recover clean anatomy in a single forward pass, eliminating stochastic sampling. Finally, a Semantic-Structural Alignment (SSA) module anchors the solution using priors from medical foundation models (MedDINOv3), ensuring clinical plausibility. Experiments on synthetic and multi-center clinical datasets show PGMP outperforms state-of-the-art methods on unseen anatomy, setting new benchmarks in efficiency and diagnostic reliability. Code and data: https://github.com/ricoleehduu/PGMP
Diffusion Model Regularized Implicit Neural Representation for CT Metal Artifact Reduction
Dec 09, 2025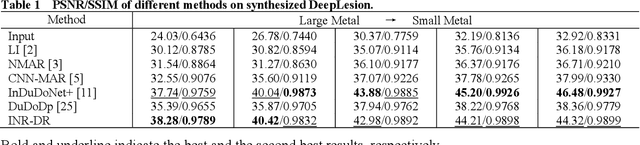
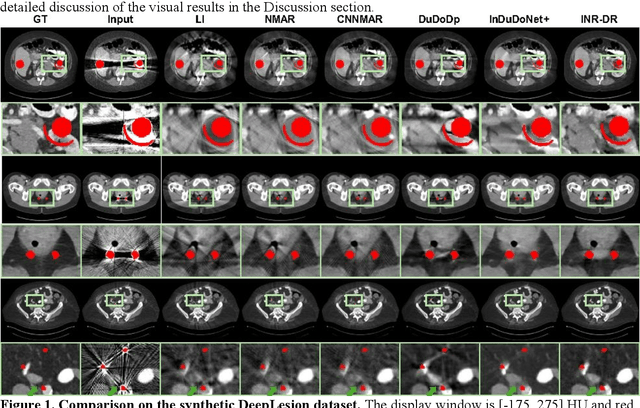
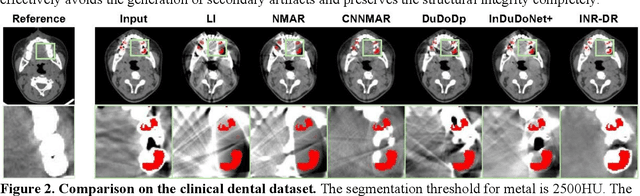
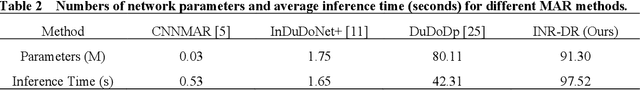
Computed tomography (CT) images are often severely corrupted by artifacts in the presence of metals. Existing supervised metal artifact reduction (MAR) approaches suffer from performance instability on known data due to their reliance on limited paired metal-clean data, which limits their clinical applicability. Moreover, existing unsupervised methods face two main challenges: 1) the CT physical geometry is not effectively incorporated into the MAR process to ensure data fidelity; 2) traditional heuristics regularization terms cannot fully capture the abundant prior knowledge available. To overcome these shortcomings, we propose diffusion model regularized implicit neural representation framework for MAR. The implicit neural representation integrates physical constraints and imposes data fidelity, while the pre-trained diffusion model provides prior knowledge to regularize the solution. Experimental results on both simulated and clinical data demonstrate the effectiveness and generalization ability of our method, highlighting its potential to be applied to clinical settings.
ReMAR-DS: Recalibrated Feature Learning for Metal Artifact Reduction and CT Domain Transformation
Jun 24, 2025Artifacts in kilo-Voltage CT (kVCT) imaging degrade image quality, impacting clinical decisions. We propose a deep learning framework for metal artifact reduction (MAR) and domain transformation from kVCT to Mega-Voltage CT (MVCT). The proposed framework, ReMAR-DS, utilizes an encoder-decoder architecture with enhanced feature recalibration, effectively reducing artifacts while preserving anatomical structures. This ensures that only relevant information is utilized in the reconstruction process. By infusing recalibrated features from the encoder block, the model focuses on relevant spatial regions (e.g., areas with artifacts) and highlights key features across channels (e.g., anatomical structures), leading to improved reconstruction of artifact-corrupted regions. Unlike traditional MAR methods, our approach bridges the gap between high-resolution kVCT and artifact-resistant MVCT, enhancing radiotherapy planning. It produces high-quality MVCT-like reconstructions, validated through qualitative and quantitative evaluations. Clinically, this enables oncologists to rely on kVCT alone, reducing repeated high-dose MVCT scans and lowering radiation exposure for cancer patients.
Prompt Guiding Multi-Scale Adaptive Sparse Representation-driven Network for Low-Dose CT MAR
Apr 28, 2025Low-dose CT (LDCT) is capable of reducing X-ray radiation exposure, but it will potentially degrade image quality, even yields metal artifacts at the case of metallic implants. For simultaneous LDCT reconstruction and metal artifact reduction (LDMAR), existing deep learning-based efforts face two main limitations: i) the network design neglects multi-scale and within-scale information; ii) training a distinct model for each dose necessitates significant storage space for multiple doses. To fill these gaps, we propose a prompt guiding multi-scale adaptive sparse representation-driven network, abbreviated as PMSRNet, for LDMAR task. Specifically, we construct PMSRNet inspired from multi-scale sparsifying frames, and it can simultaneously employ within-scale characteristics and cross-scale complementarity owing to an elaborated prompt guiding scale-adaptive threshold generator (PSATG) and a built multi-scale coefficient fusion module (MSFuM). The PSATG can adaptively capture multiple contextual information to generate more faithful thresholds, achieved by fusing features from local, regional, and global levels. Furthermore, we elaborate a model interpretable dual domain LDMAR framework called PDuMSRNet, and train single model with a prompt guiding strategy for multiple dose levels. We build a prompt guiding module, whose input contains dose level, metal mask and input instance, to provide various guiding information, allowing a single model to accommodate various CT dose settings. Extensive experiments at various dose levels demonstrate that the proposed methods outperform the state-of-the-art LDMAR methods.
Radiologist-in-the-Loop Self-Training for Generalizable CT Metal Artifact Reduction
Jan 26, 2025



Metal artifacts in computed tomography (CT) images can significantly degrade image quality and impede accurate diagnosis. Supervised metal artifact reduction (MAR) methods, trained using simulated datasets, often struggle to perform well on real clinical CT images due to a substantial domain gap. Although state-of-the-art semi-supervised methods use pseudo ground-truths generated by a prior network to mitigate this issue, their reliance on a fixed prior limits both the quality and quantity of these pseudo ground-truths, introducing confirmation bias and reducing clinical applicability. To address these limitations, we propose a novel Radiologist-In-the-loop SElf-training framework for MAR, termed RISE-MAR, which can integrate radiologists' feedback into the semi-supervised learning process, progressively improving the quality and quantity of pseudo ground-truths for enhanced generalization on real clinical CT images. For quality assurance, we introduce a clinical quality assessor model that emulates radiologist evaluations, effectively selecting high-quality pseudo ground-truths for semi-supervised training. For quantity assurance, our self-training framework iteratively generates additional high-quality pseudo ground-truths, expanding the clinical dataset and further improving model generalization. Extensive experimental results on multiple clinical datasets demonstrate the superior generalization performance of our RISE-MAR over state-of-the-art methods, advancing the development of MAR models for practical application. Code is available at https://github.com/Masaaki-75/rise-mar.
B1+ mapping near metallic implants using turbo spin echo pulse sequences
Jan 10, 2025



Purpose: To propose a B1+ mapping technique for imaging of body parts containing metal hardware, based on magnitude images acquired with turbo spin echo (TSE) pulse sequences. Theory and Methods: To encode the underlying B1+, multiple (two to four) TSE image sets with various excitation and refocusing flip angles were acquired. To this end, the acquired signal intensities were matched to a database of simulated signals which was generated by solving the Bloch equations taking into account the exact sequence parameters. The retrieved B1+ values were validated against gradient-recalled and spin echo dual angle methods, as well as a vendor-provided turboFLASH-based mapping sequence, in gel phantoms and human subjects without and with metal implants. Results: In the absence of metal, phantom experiments demonstrated excellent agreement between the proposed technique using three or four flip angle sets and reference dual angle methods. In human subjects without metal implants, the proposed technique with three or four flip angle sets showed excellent correlation with the spin echo dual angle method. In the presence of metal, both phantoms and human subjects revealed a narrow range of B1+ estimation with the reference techniques, whereas the proposed technique successfully resolved B1+ near the metal. In select cases, the technique was implemented in conjunction with multispectral metal artifact reduction sequences and successfully applied for B1+ shimming. Conclusion: The proposed technique enables resolution of B1+ values in regions near metal hardware, overcoming susceptibility-related and narrow-range limitations of standard mapping techniques.
Photon-Counting CT in Cancer Radiotherapy: Technological Advances and Clinical Benefits
Oct 26, 2024



Photon-counting computed tomography (PCCT) marks a significant advancement over conventional energy-integrating detector (EID) CT systems. This review highlights PCCT's superior spatial and contrast resolution, reduced radiation dose, and multi-energy imaging capabilities, which address key challenges in radiotherapy, such as accurate tumor delineation, precise dose calculation, and treatment response monitoring. PCCT's improved anatomical clarity enhances tumor targeting while minimizing damage to surrounding healthy tissues. Additionally, metal artifact reduction (MAR) and quantitative imaging capabilities optimize workflows, enabling adaptive radiotherapy and radiomics-driven personalized treatment. Emerging clinical applications in brachytherapy and radiopharmaceutical therapy (RPT) show promising outcomes, although challenges like high costs and limited software integration remain. With advancements in artificial intelligence (AI) and dedicated radiotherapy packages, PCCT is poised to transform precision, safety, and efficacy in cancer radiotherapy, marking it as a pivotal technology for future clinical practice.
Unlocking the Potential of Early Epochs: Uncertainty-aware CT Metal Artifact Reduction
Jun 18, 2024In computed tomography (CT), the presence of metallic implants in patients often leads to disruptive artifacts in the reconstructed images, hindering accurate diagnosis. Recently, a large amount of supervised deep learning-based approaches have been proposed for metal artifact reduction (MAR). However, these methods neglect the influence of initial training weights. In this paper, we have discovered that the uncertainty image computed from the restoration result of initial training weights can effectively highlight high-frequency regions, including metal artifacts. This observation can be leveraged to assist the MAR network in removing metal artifacts. Therefore, we propose an uncertainty constraint (UC) loss that utilizes the uncertainty image as an adaptive weight to guide the MAR network to focus on the metal artifact region, leading to improved restoration. The proposed UC loss is designed to be a plug-and-play method, compatible with any MAR framework, and easily adoptable. To validate the effectiveness of the UC loss, we conduct extensive experiments on the public available Deeplesion and CLINIC-metal dataset. Experimental results demonstrate that the UC loss further optimizes the network training process and significantly improves the removal of metal artifacts.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge