Mammogram
Papers and Code
MGRegBench: A Novel Benchmark Dataset with Anatomical Landmarks for Mammography Image Registration
Dec 19, 2025Robust mammography registration is essential for clinical applications like tracking disease progression and monitoring longitudinal changes in breast tissue. However, progress has been limited by the absence of public datasets and standardized benchmarks. Existing studies are often not directly comparable, as they use private data and inconsistent evaluation frameworks. To address this, we present MGRegBench, a public benchmark dataset for mammogram registration. It comprises over 5,000 image pairs, with 100 containing manual anatomical landmarks and segmentation masks for rigorous evaluation. This makes MGRegBench one of the largest public 2D registration datasets with manual annotations. Using this resource, we benchmarked diverse registration methods including classical (ANTs), learning-based (VoxelMorph, TransMorph), implicit neural representation (IDIR), a classic mammography-specific approach, and a recent state-of-the-art deep learning method MammoRegNet. The implementations were adapted to this modality from the authors' implementations or re-implemented from scratch. Our contributions are: (1) the first public dataset of this scale with manual landmarks and masks for mammography registration; (2) the first like-for-like comparison of diverse methods on this modality; and (3) an extensive analysis of deep learning-based registration. We publicly release our code and data to establish a foundational resource for fair comparisons and catalyze future research. The source code and data are at https://github.com/KourtKardash/MGRegBench.
A Bayesian Model for Multi-stage Censoring
Nov 18, 2025



Many sequential decision settings in healthcare feature funnel structures characterized by a series of stages, such as screenings or evaluations, where the number of patients who advance to each stage progressively decreases and decisions become increasingly costly. For example, an oncologist may first conduct a breast exam, followed by a mammogram for patients with concerning exams, followed by a biopsy for patients with concerning mammograms. A key challenge is that the ground truth outcome, such as the biopsy result, is only revealed at the end of this funnel. The selective censoring of the ground truth can introduce statistical biases in risk estimation, especially in underserved patient groups, whose outcomes are more frequently censored. We develop a Bayesian model for funnel decision structures, drawing from prior work on selective labels and censoring. We first show in synthetic settings that our model is able to recover the true parameters and predict outcomes for censored patients more accurately than baselines. We then apply our model to a dataset of emergency department visits, where in-hospital mortality is observed only for those who are admitted to either the hospital or ICU. We find that there are gender-based differences in hospital and ICU admissions. In particular, our model estimates that the mortality risk threshold to admit women to the ICU is higher for women (5.1%) than for men (4.5%).
The Impact of Longitudinal Mammogram Alignment on Breast Cancer Risk Assessment
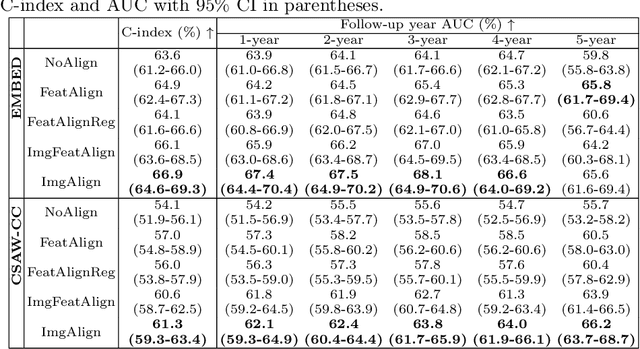
Nov 11, 2025Regular mammography screening is crucial for early breast cancer detection. By leveraging deep learning-based risk models, screening intervals can be personalized, especially for high-risk individuals. While recent methods increasingly incorporate longitudinal information from prior mammograms, accurate spatial alignment across time points remains a key challenge. Misalignment can obscure meaningful tissue changes and degrade model performance. In this study, we provide insights into various alignment strategies, image-based registration, feature-level (representation space) alignment with and without regularization, and implicit alignment methods, for their effectiveness in longitudinal deep learning-based risk modeling. Using two large-scale mammography datasets, we assess each method across key metrics, including predictive accuracy, precision, recall, and deformation field quality. Our results show that image-based registration consistently outperforms the more recently favored feature-based and implicit approaches across all metrics, enabling more accurate, temporally consistent predictions and generating smooth, anatomically plausible deformation fields. Although regularizing the deformation field improves deformation quality, it reduces the risk prediction performance of feature-level alignment. Applying image-based deformation fields within the feature space yields the best risk prediction performance. These findings underscore the importance of image-based deformation fields for spatial alignment in longitudinal risk modeling, offering improved prediction accuracy and robustness. This approach has strong potential to enhance personalized screening and enable earlier interventions for high-risk individuals. The code is available at https://github.com/sot176/Mammogram_Alignment_Study_Risk_Prediction.git, allowing full reproducibility of the results.
MV-MLM: Bridging Multi-View Mammography and Language for Breast Cancer Diagnosis and Risk Prediction
Oct 30, 2025



Large annotated datasets are essential for training robust Computer-Aided Diagnosis (CAD) models for breast cancer detection or risk prediction. However, acquiring such datasets with fine-detailed annotation is both costly and time-consuming. Vision-Language Models (VLMs), such as CLIP, which are pre-trained on large image-text pairs, offer a promising solution by enhancing robustness and data efficiency in medical imaging tasks. This paper introduces a novel Multi-View Mammography and Language Model for breast cancer classification and risk prediction, trained on a dataset of paired mammogram images and synthetic radiology reports. Our MV-MLM leverages multi-view supervision to learn rich representations from extensive radiology data by employing cross-modal self-supervision across image-text pairs. This includes multiple views and the corresponding pseudo-radiology reports. We propose a novel joint visual-textual learning strategy to enhance generalization and accuracy performance over different data types and tasks to distinguish breast tissues or cancer characteristics(calcification, mass) and utilize these patterns to understand mammography images and predict cancer risk. We evaluated our method on both private and publicly available datasets, demonstrating that the proposed model achieves state-of-the-art performance in three classification tasks: (1) malignancy classification, (2) subtype classification, and (3) image-based cancer risk prediction. Furthermore, the model exhibits strong data efficiency, outperforming existing fully supervised or VLM baselines while trained on synthetic text reports and without the need for actual radiology reports.
$Δ$t-Mamba3D: A Time-Aware Spatio-Temporal State-Space Model for Breast Cancer Risk Prediction
Oct 21, 2025



Longitudinal analysis of sequential radiological images is hampered by a fundamental data challenge: how to effectively model a sequence of high-resolution images captured at irregular time intervals. This data structure contains indispensable spatial and temporal cues that current methods fail to fully exploit. Models often compromise by either collapsing spatial information into vectors or applying spatio-temporal models that are computationally inefficient and incompatible with non-uniform time steps. We address this challenge with Time-Aware $\Delta$t-Mamba3D, a novel state-space architecture adapted for longitudinal medical imaging. Our model simultaneously encodes irregular inter-visit intervals and rich spatio-temporal context while remaining computationally efficient. Its core innovation is a continuous-time selective scanning mechanism that explicitly integrates the true time difference between exams into its state transitions. This is complemented by a multi-scale 3D neighborhood fusion module that robustly captures spatio-temporal relationships. In a comprehensive breast cancer risk prediction benchmark using sequential screening mammogram exams, our model shows superior performance, improving the validation c-index by 2-5 percentage points and achieving higher 1-5 year AUC scores compared to established variants of recurrent, transformer, and state-space models. Thanks to its linear complexity, the model can efficiently process long and complex patient screening histories of mammograms, forming a new framework for longitudinal image analysis.
Bidirectional Mammogram View Translation with Column-Aware and Implicit 3D Conditional Diffusion
Oct 06, 2025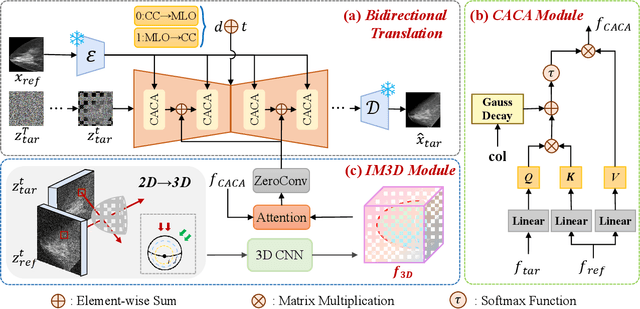
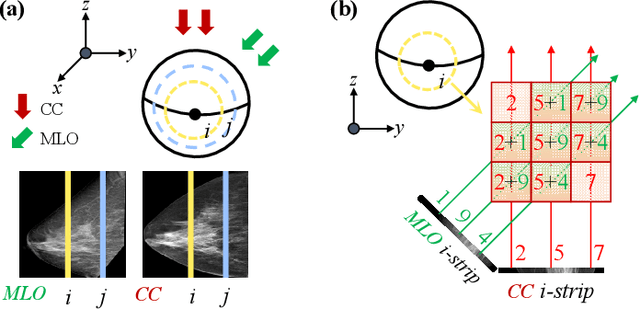
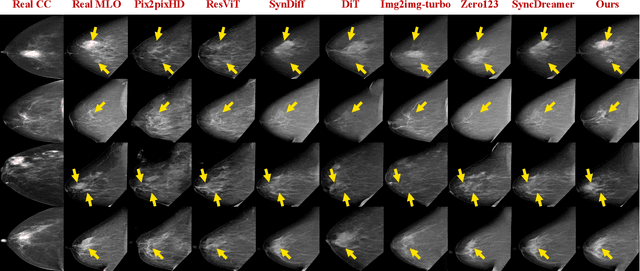
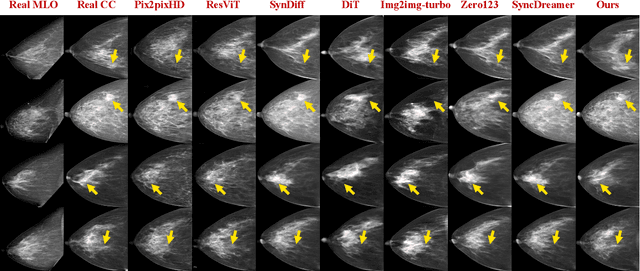
Dual-view mammography, including craniocaudal (CC) and mediolateral oblique (MLO) projections, offers complementary anatomical views crucial for breast cancer diagnosis. However, in real-world clinical workflows, one view may be missing, corrupted, or degraded due to acquisition errors or compression artifacts, limiting the effectiveness of downstream analysis. View-to-view translation can help recover missing views and improve lesion alignment. Unlike natural images, this task in mammography is highly challenging due to large non-rigid deformations and severe tissue overlap in X-ray projections, which obscure pixel-level correspondences. In this paper, we propose Column-Aware and Implicit 3D Diffusion (CA3D-Diff), a novel bidirectional mammogram view translation framework based on conditional diffusion model. To address cross-view structural misalignment, we first design a column-aware cross-attention mechanism that leverages the geometric property that anatomically corresponding regions tend to lie in similar column positions across views. A Gaussian-decayed bias is applied to emphasize local column-wise correlations while suppressing distant mismatches. Furthermore, we introduce an implicit 3D structure reconstruction module that back-projects noisy 2D latents into a coarse 3D feature volume based on breast-view projection geometry. The reconstructed 3D structure is refined and injected into the denoising UNet to guide cross-view generation with enhanced anatomical awareness. Extensive experiments demonstrate that CA3D-Diff achieves superior performance in bidirectional tasks, outperforming state-of-the-art methods in visual fidelity and structural consistency. Furthermore, the synthesized views effectively improve single-view malignancy classification in screening settings, demonstrating the practical value of our method in real-world diagnostics.
Mammo-Mamba: A Hybrid State-Space and Transformer Architecture with Sequential Mixture of Experts for Multi-View Mammography
Jul 23, 2025Breast cancer (BC) remains one of the leading causes of cancer-related mortality among women, despite recent advances in Computer-Aided Diagnosis (CAD) systems. Accurate and efficient interpretation of multi-view mammograms is essential for early detection, driving a surge of interest in Artificial Intelligence (AI)-powered CAD models. While state-of-the-art multi-view mammogram classification models are largely based on Transformer architectures, their computational complexity scales quadratically with the number of image patches, highlighting the need for more efficient alternatives. To address this challenge, we propose Mammo-Mamba, a novel framework that integrates Selective State-Space Models (SSMs), transformer-based attention, and expert-driven feature refinement into a unified architecture. Mammo-Mamba extends the MambaVision backbone by introducing the Sequential Mixture of Experts (SeqMoE) mechanism through its customized SecMamba block. The SecMamba is a modified MambaVision block that enhances representation learning in high-resolution mammographic images by enabling content-adaptive feature refinement. These blocks are integrated into the deeper stages of MambaVision, allowing the model to progressively adjust feature emphasis through dynamic expert gating, effectively mitigating the limitations of traditional Transformer models. Evaluated on the CBIS-DDSM benchmark dataset, Mammo-Mamba achieves superior classification performance across all key metrics while maintaining computational efficiency.
A Hybrid CNN-VSSM model for Multi-View, Multi-Task Mammography Analysis: Robust Diagnosis with Attention-Based Fusion
Jul 22, 2025Early and accurate interpretation of screening mammograms is essential for effective breast cancer detection, yet it remains a complex challenge due to subtle imaging findings and diagnostic ambiguity. Many existing AI approaches fall short by focusing on single view inputs or single-task outputs, limiting their clinical utility. To address these limitations, we propose a novel multi-view, multitask hybrid deep learning framework that processes all four standard mammography views and jointly predicts diagnostic labels and BI-RADS scores for each breast. Our architecture integrates a hybrid CNN VSSM backbone, combining convolutional encoders for rich local feature extraction with Visual State Space Models (VSSMs) to capture global contextual dependencies. To improve robustness and interpretability, we incorporate a gated attention-based fusion module that dynamically weights information across views, effectively handling cases with missing data. We conduct extensive experiments across diagnostic tasks of varying complexity, benchmarking our proposed hybrid models against baseline CNN architectures and VSSM models in both single task and multi task learning settings. Across all tasks, the hybrid models consistently outperform the baselines. In the binary BI-RADS 1 vs. 5 classification task, the shared hybrid model achieves an AUC of 0.9967 and an F1 score of 0.9830. For the more challenging ternary classification, it attains an F1 score of 0.7790, while in the five-class BI-RADS task, the best F1 score reaches 0.4904. These results highlight the effectiveness of the proposed hybrid framework and underscore both the potential and limitations of multitask learning for improving diagnostic performance and enabling clinically meaningful mammography analysis.
Reconsidering Explicit Longitudinal Mammography Alignment for Enhanced Breast Cancer Risk Prediction
Jun 24, 2025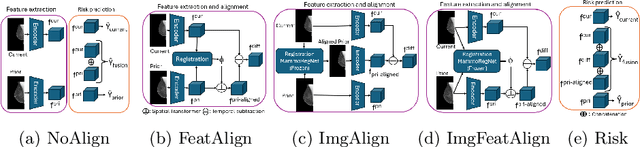
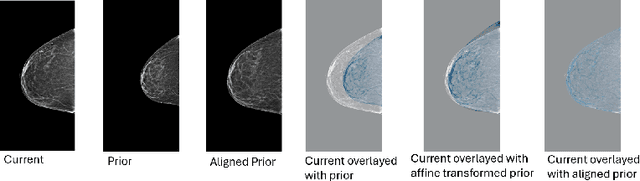

Regular mammography screening is essential for early breast cancer detection. Deep learning-based risk prediction methods have sparked interest to adjust screening intervals for high-risk groups. While early methods focused only on current mammograms, recent approaches leverage the temporal aspect of screenings to track breast tissue changes over time, requiring spatial alignment across different time points. Two main strategies for this have emerged: explicit feature alignment through deformable registration and implicit learned alignment using techniques like transformers, with the former providing more control. However, the optimal approach for explicit alignment in mammography remains underexplored. In this study, we provide insights into where explicit alignment should occur (input space vs. representation space) and if alignment and risk prediction should be jointly optimized. We demonstrate that jointly learning explicit alignment in representation space while optimizing risk estimation performance, as done in the current state-of-the-art approach, results in a trade-off between alignment quality and predictive performance and show that image-level alignment is superior to representation-level alignment, leading to better deformation field quality and enhanced risk prediction accuracy. The code is available at https://github.com/sot176/Longitudinal_Mammogram_Alignment.git.
MAMBO: High-Resolution Generative Approach for Mammography Images
Jun 10, 2025Mammography is the gold standard for the detection and diagnosis of breast cancer. This procedure can be significantly enhanced with Artificial Intelligence (AI)-based software, which assists radiologists in identifying abnormalities. However, training AI systems requires large and diverse datasets, which are often difficult to obtain due to privacy and ethical constraints. To address this issue, the paper introduces MAMmography ensemBle mOdel (MAMBO), a novel patch-based diffusion approach designed to generate full-resolution mammograms. Diffusion models have shown breakthrough results in realistic image generation, yet few studies have focused on mammograms, and none have successfully generated high-resolution outputs required to capture fine-grained features of small lesions. To achieve this, MAMBO integrates separate diffusion models to capture both local and global (image-level) contexts. The contextual information is then fed into the final patch-based model, significantly aiding the noise removal process. This thoughtful design enables MAMBO to generate highly realistic mammograms of up to 3840x3840 pixels. Importantly, this approach can be used to enhance the training of classification models and extended to anomaly detection. Experiments, both numerical and radiologist validation, assess MAMBO's capabilities in image generation, super-resolution, and anomaly detection, highlighting its potential to enhance mammography analysis for more accurate diagnoses and earlier lesion detection.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge