Xiaosong Wang
Text-guided Foundation Model Adaptation for Pathological Image Classification
Jul 27, 2023



Abstract:The recent surge of foundation models in computer vision and natural language processing opens up perspectives in utilizing multi-modal clinical data to train large models with strong generalizability. Yet pathological image datasets often lack biomedical text annotation and enrichment. Guiding data-efficient image diagnosis from the use of biomedical text knowledge becomes a substantial interest. In this paper, we propose to Connect Image and Text Embeddings (CITE) to enhance pathological image classification. CITE injects text insights gained from language models pre-trained with a broad range of biomedical texts, leading to adapt foundation models towards pathological image understanding. Through extensive experiments on the PatchGastric stomach tumor pathological image dataset, we demonstrate that CITE achieves leading performance compared with various baselines especially when training data is scarce. CITE offers insights into leveraging in-domain text knowledge to reinforce data-efficient pathological image classification. Code is available at https://github.com/Yunkun-Zhang/CITE.
Exploring Data Redundancy in Real-world Image Classification through Data Selection
Jun 25, 2023



Abstract:Deep learning models often require large amounts of data for training, leading to increased costs. It is particularly challenging in medical imaging, i.e., gathering distributed data for centralized training, and meanwhile, obtaining quality labels remains a tedious job. Many methods have been proposed to address this issue in various training paradigms, e.g., continual learning, active learning, and federated learning, which indeed demonstrate certain forms of the data valuation process. However, existing methods are either overly intuitive or limited to common clean/toy datasets in the experiments. In this work, we present two data valuation metrics based on Synaptic Intelligence and gradient norms, respectively, to study the redundancy in real-world image data. Novel online and offline data selection algorithms are then proposed via clustering and grouping based on the examined data values. Our online approach effectively evaluates data utilizing layerwise model parameter updates and gradients in each epoch and can accelerate model training with fewer epochs and a subset (e.g., 19%-59%) of data while maintaining equivalent levels of accuracy in a variety of datasets. It also extends to the offline coreset construction, producing subsets of only 18%-30% of the original. The codes for the proposed adaptive data selection and coreset computation are available (https://github.com/ZhenyuTANG2023/data_selection).
MedFMC: A Real-world Dataset and Benchmark For Foundation Model Adaptation in Medical Image Classification
Jun 16, 2023Abstract:Foundation models, often pre-trained with large-scale data, have achieved paramount success in jump-starting various vision and language applications. Recent advances further enable adapting foundation models in downstream tasks efficiently using only a few training samples, e.g., in-context learning. Yet, the application of such learning paradigms in medical image analysis remains scarce due to the shortage of publicly accessible data and benchmarks. In this paper, we aim at approaches adapting the foundation models for medical image classification and present a novel dataset and benchmark for the evaluation, i.e., examining the overall performance of accommodating the large-scale foundation models downstream on a set of diverse real-world clinical tasks. We collect five sets of medical imaging data from multiple institutes targeting a variety of real-world clinical tasks (22,349 images in total), i.e., thoracic diseases screening in X-rays, pathological lesion tissue screening, lesion detection in endoscopy images, neonatal jaundice evaluation, and diabetic retinopathy grading. Results of multiple baseline methods are demonstrated using the proposed dataset from both accuracy and cost-effective perspectives.
T-AutoML: Automated Machine Learning for Lesion Segmentation using Transformers in 3D Medical Imaging
Nov 15, 2021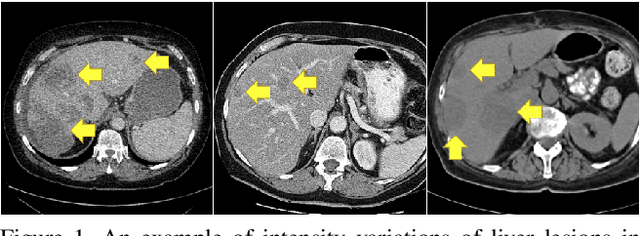
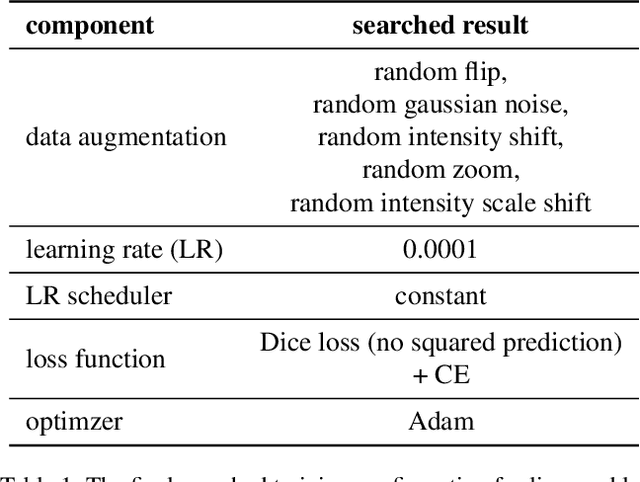
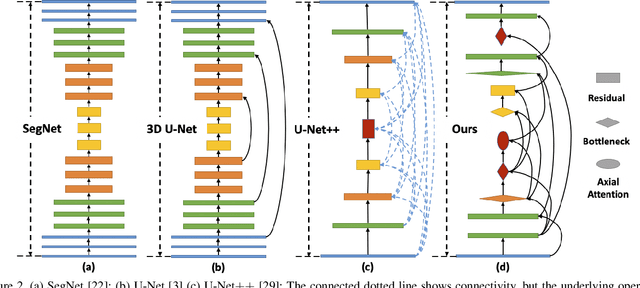
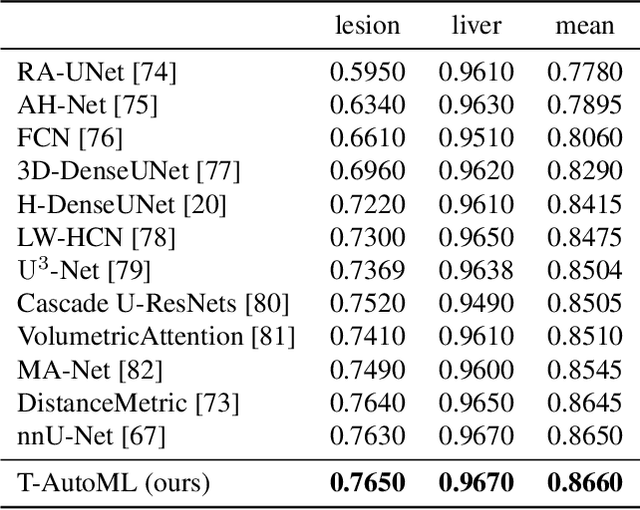
Abstract:Lesion segmentation in medical imaging has been an important topic in clinical research. Researchers have proposed various detection and segmentation algorithms to address this task. Recently, deep learning-based approaches have significantly improved the performance over conventional methods. However, most state-of-the-art deep learning methods require the manual design of multiple network components and training strategies. In this paper, we propose a new automated machine learning algorithm, T-AutoML, which not only searches for the best neural architecture, but also finds the best combination of hyper-parameters and data augmentation strategies simultaneously. The proposed method utilizes the modern transformer model, which is introduced to adapt to the dynamic length of the search space embedding and can significantly improve the ability of the search. We validate T-AutoML on several large-scale public lesion segmentation data-sets and achieve state-of-the-art performance.
Improving Pneumonia Localization via Cross-Attention on Medical Images and Reports
Oct 06, 2021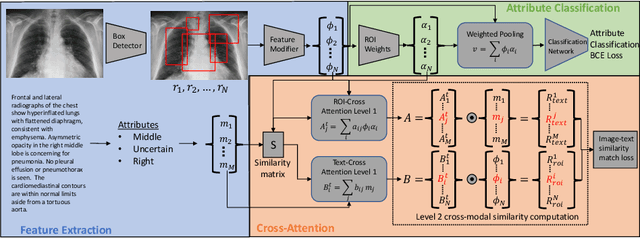
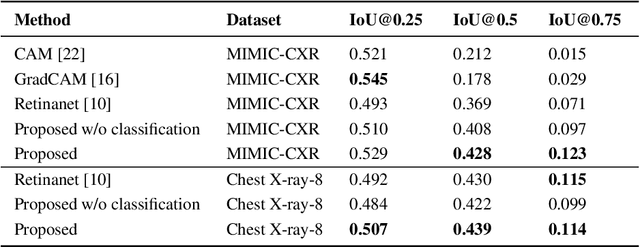
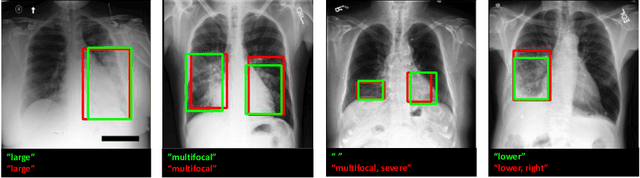
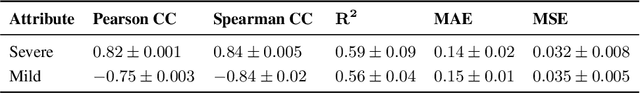
Abstract:Localization and characterization of diseases like pneumonia are primary steps in a clinical pipeline, facilitating detailed clinical diagnosis and subsequent treatment planning. Additionally, such location annotated datasets can provide a pathway for deep learning models to be used for downstream tasks. However, acquiring quality annotations is expensive on human resources and usually requires domain expertise. On the other hand, medical reports contain a plethora of information both about pneumonia characteristics and its location. In this paper, we propose a novel weakly-supervised attention-driven deep learning model that leverages encoded information in medical reports during training to facilitate better localization. Our model also performs classification of attributes that are associated to pneumonia and extracted from medical reports for supervision. Both the classification and localization are trained in conjunction and once trained, the model can be utilized for both the localization and characterization of pneumonia using only the input image. In this paper, we explore and analyze the model using chest X-ray datasets and demonstrate qualitatively and quantitatively that the introduction of textual information improves pneumonia localization. We showcase quantitative results on two datasets, MIMIC-CXR and Chest X-ray-8, and we also showcase severity characterization on the COVID-19 dataset.
Federated Whole Prostate Segmentation in MRI with Personalized Neural Architectures
Jul 16, 2021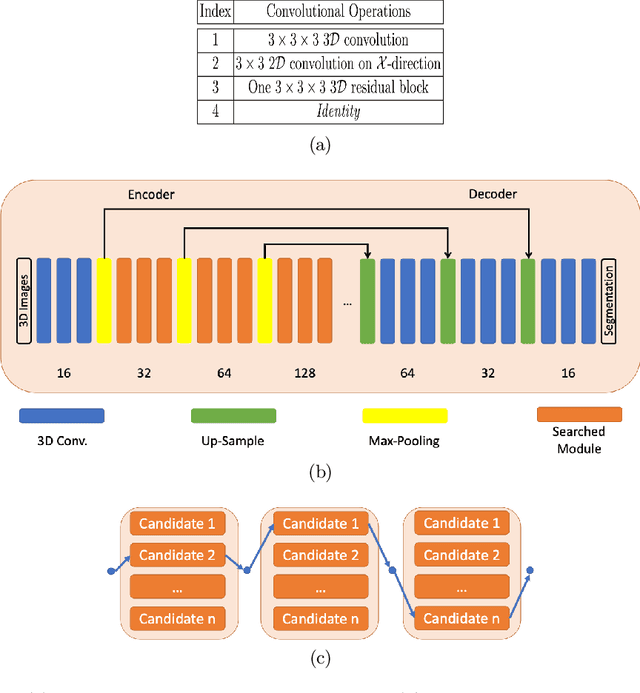
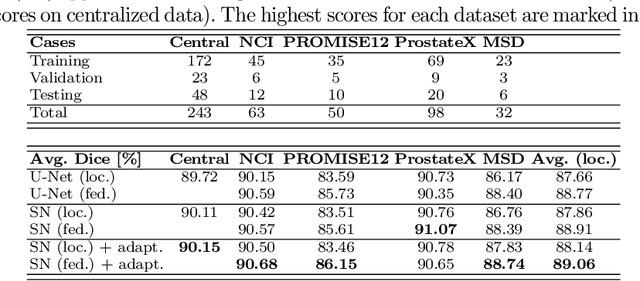
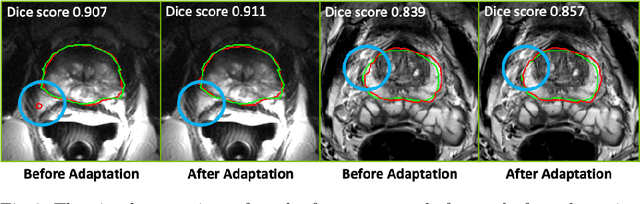
Abstract:Building robust deep learning-based models requires diverse training data, ideally from several sources. However, these datasets cannot be combined easily because of patient privacy concerns or regulatory hurdles, especially if medical data is involved. Federated learning (FL) is a way to train machine learning models without the need for centralized datasets. Each FL client trains on their local data while only sharing model parameters with a global server that aggregates the parameters from all clients. At the same time, each client's data can exhibit differences and inconsistencies due to the local variation in the patient population, imaging equipment, and acquisition protocols. Hence, the federated learned models should be able to adapt to the local particularities of a client's data. In this work, we combine FL with an AutoML technique based on local neural architecture search by training a "supernet". Furthermore, we propose an adaptation scheme to allow for personalized model architectures at each FL client's site. The proposed method is evaluated on four different datasets from 3D prostate MRI and shown to improve the local models' performance after adaptation through selecting an optimal path through the AutoML supernet.
Self-supervised Image-text Pre-training With Mixed Data In Chest X-rays
Mar 30, 2021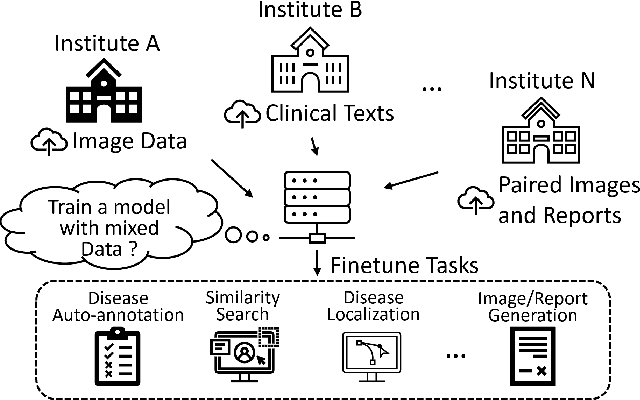
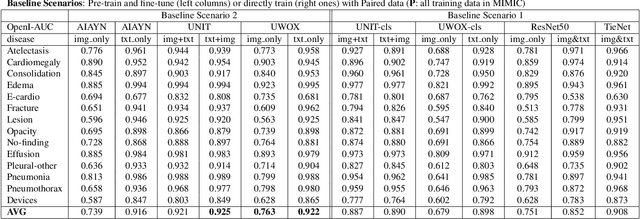
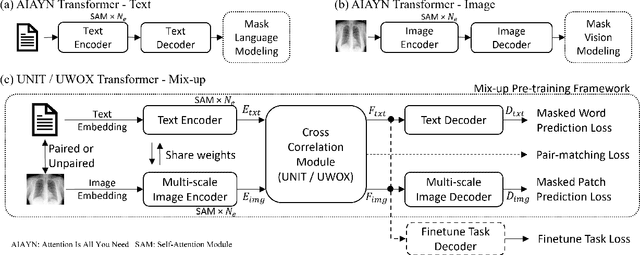
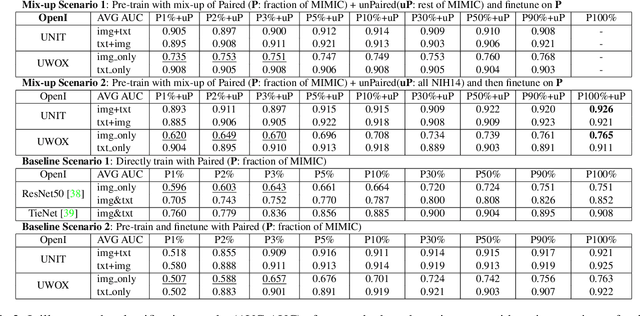
Abstract:Pre-trained models, e.g., from ImageNet, have proven to be effective in boosting the performance of many downstream applications. It is too demanding to acquire large-scale annotations to build such models for medical imaging. Meanwhile, there are numerous clinical data (in the form of images and text reports) stored in the hospital information systems. The paired image-text data from the same patient study could be utilized for the pre-training task in a weakly supervised manner. However, the integrity, accessibility, and amount of such raw data vary across different institutes, e.g., paired vs. unpaired (image-only or text-only). In this work, we introduce an image-text pre-training framework that can learn from these raw data with mixed data inputs, i.e., paired image-text data, a mixture of paired and unpaired data. The unpaired data can be sourced from one or multiple institutes (e.g., images from one institute coupled with texts from another). Specifically, we propose a transformer-based training framework for jointly learning the representation of both the image and text data. In addition to the existing masked language modeling, multi-scale masked vision modeling is introduced as a self-supervised training task for image patch regeneration. We not only demonstrate the feasibility of pre-training across mixed data inputs but also illustrate the benefits of adopting such pre-trained models in 3 chest X-ray applications, i.e., classification, retrieval, and image regeneration. Superior results are reported in comparison to prior art using MIMIC-CXR, NIH14-CXR, and OpenI-CXR datasets.
Transformer Query-Target Knowledge Discovery (TEND): Drug Discovery from CORD-19
Dec 11, 2020
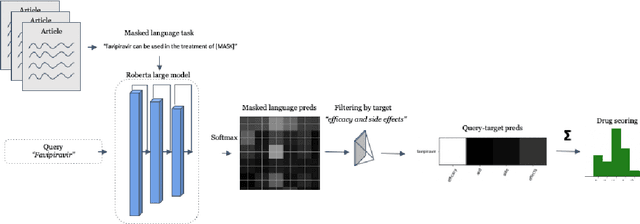
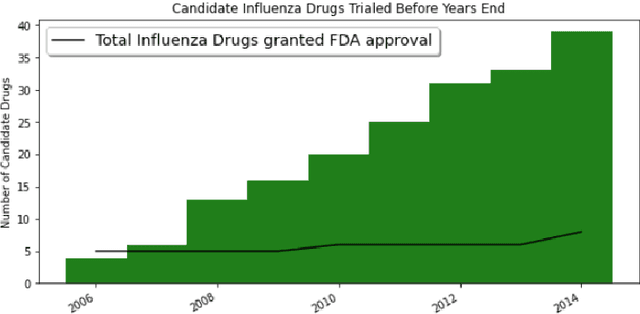
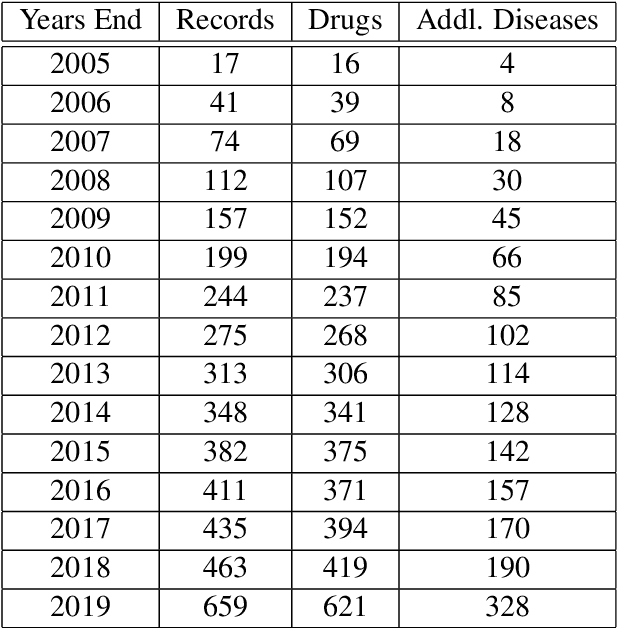
Abstract:Previous work established skip-gram word2vec models could be used to mine knowledge in the materials science literature for the discovery of thermoelectrics. Recent transformer architectures have shown great progress in language modeling and associated fine-tuned tasks, but they have yet to be adapted for drug discovery. We present a RoBERTa transformer-based method that extends the masked language token prediction using query-target conditioning to treat the specificity challenge. The transformer discovery method entails several benefits over the word2vec method including domain-specific (antiviral) analogy performance, negation handling, and flexible query analysis (specific) and is demonstrated on influenza drug discovery. To stimulate COVID-19 research, we release an influenza clinical trials and antiviral analogies dataset used in conjunction with the COVID-19 Open Research Dataset Challenge (CORD-19) literature dataset in the study. We examine k-shot fine-tuning to improve the downstream analogies performance as well as to mine analogies for model explainability. Further, the query-target analysis is verified in a forward chaining analysis against the influenza drug clinical trials dataset, before adapted for COVID-19 drugs (combinations and side-effects) and on-going clinical trials. In consideration of the present topic, we release the model, dataset, and code.
Federated Semi-Supervised Learning for COVID Region Segmentation in Chest CT using Multi-National Data from China, Italy, Japan
Nov 23, 2020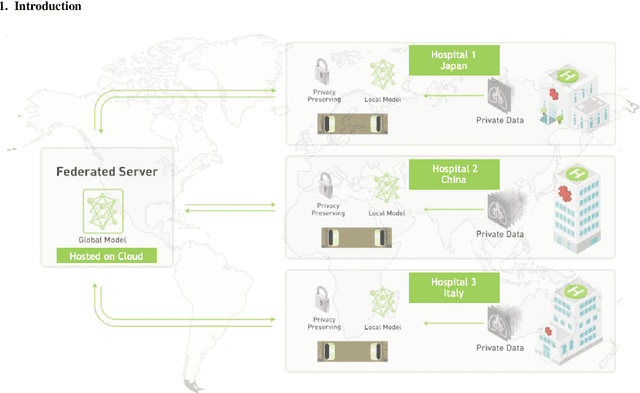
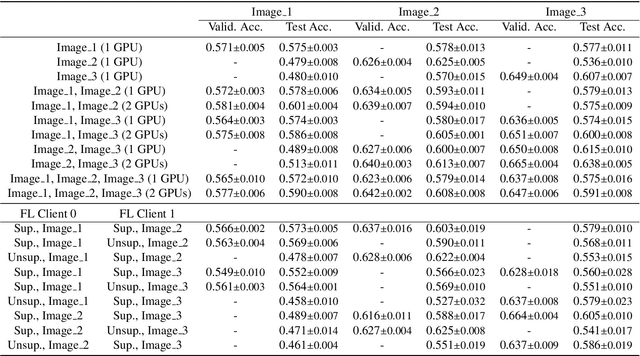
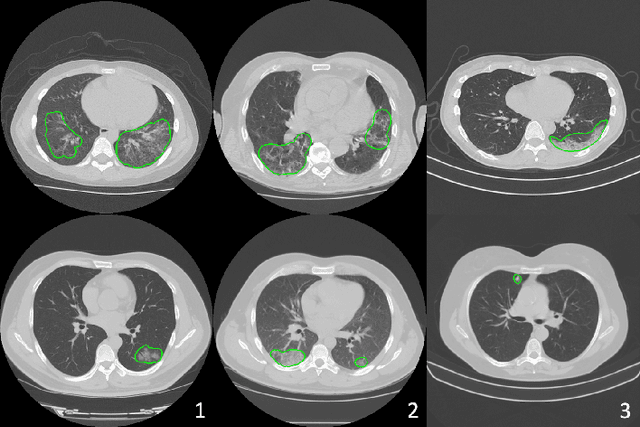
Abstract:The recent outbreak of COVID-19 has led to urgent needs for reliable diagnosis and management of SARS-CoV-2 infection. As a complimentary tool, chest CT has been shown to be able to reveal visual patterns characteristic for COVID-19, which has definite value at several stages during the disease course. To facilitate CT analysis, recent efforts have focused on computer-aided characterization and diagnosis, which has shown promising results. However, domain shift of data across clinical data centers poses a serious challenge when deploying learning-based models. In this work, we attempt to find a solution for this challenge via federated and semi-supervised learning. A multi-national database consisting of 1704 scans from three countries is adopted to study the performance gap, when training a model with one dataset and applying it to another. Expert radiologists manually delineated 945 scans for COVID-19 findings. In handling the variability in both the data and annotations, a novel federated semi-supervised learning technique is proposed to fully utilize all available data (with or without annotations). Federated learning avoids the need for sensitive data-sharing, which makes it favorable for institutions and nations with strict regulatory policy on data privacy. Moreover, semi-supervision potentially reduces the annotation burden under a distributed setting. The proposed framework is shown to be effective compared to fully supervised scenarios with conventional data sharing instead of model weight sharing.
Learning Image Labels On-the-fly for Training Robust Classification Models
Oct 02, 2020
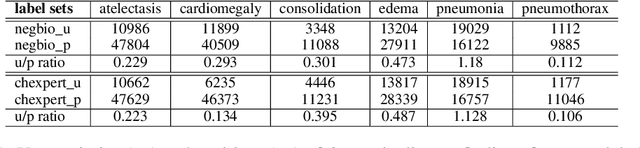
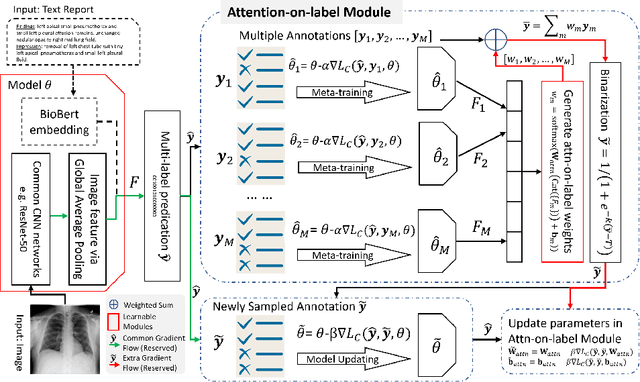
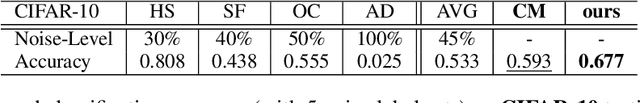
Abstract:Current deep learning paradigms largely benefit from the tremendous amount of annotated data. However, the quality of the annotations often varies among labelers. Multi-observer studies have been conducted to study these annotation variances (by labeling the same data for multiple times) and its effects on critical applications like medical image analysis. This process indeed adds an extra burden to the already tedious annotation work that usually requires professional training and expertise in the specific domains. On the other hand, automated annotation methods based on NLP algorithms have recently shown promise as a reasonable alternative, relying on the existing diagnostic reports of those images that are widely available in the clinical system. Compared to human labelers, different algorithms provide labels with varying qualities that are even noisier. In this paper, we show how noisy annotations (e.g., from different algorithm-based labelers) can be utilized together and mutually benefit the learning of classification tasks. Specifically, the concept of attention-on-label is introduced to sample better label sets on-the-fly as the training data. A meta-training based label-sampling module is designed to attend the labels that benefit the model learning the most through additional back-propagation processes. We apply the attention-on-label scheme on the classification task of a synthetic noisy CIFAR-10 dataset to prove the concept, and then demonstrate superior results (3-5% increase on average in multiple disease classification AUCs) on the chest x-ray images from a hospital-scale dataset (MIMIC-CXR) and hand-labeled dataset (OpenI) in comparison to regular training paradigms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge