Xiaoying Tang
FedEBA+: Towards Fair and Effective Federated Learning via Entropy-Based Model
Jan 29, 2023Abstract:Ensuring fairness is a crucial aspect of Federated Learning (FL), which enables the model to perform consistently across all clients. However, designing an FL algorithm that simultaneously improves global model performance and promotes fairness remains a formidable challenge, as achieving the latter often necessitates a trade-off with the former.To address this challenge, we propose a new FL algorithm, FedEBA+, which enhances fairness while simultaneously improving global model performance. Our approach incorporates a fair aggregation scheme that assigns higher weights to underperforming clients and a novel model update method for FL. Besides, we show the theoretical convergence analysis and demonstrate the fairness of our algorithm. Experimental results reveal that FedEBA+ outperforms other SOTA fairness FL methods in terms of both fairness and global model's performance.
FedConceptEM: Robust Federated Learning Under Diverse Distribution Shifts
Jan 29, 2023Abstract:Federated Learning (FL) is a machine learning paradigm that protects privacy by keeping client data on edge devices. However, optimizing FL in practice can be challenging due to the diversity and heterogeneity of the learning system. Recent research efforts have aimed to improve the optimization of FL with distribution shifts, but it is still an open problem how to train FL models when multiple types of distribution shifts, i.e., feature distribution skew, label distribution skew, and concept shift occur simultaneously. To address this challenge, we propose a novel algorithm framework, FedConceptEM, for handling diverse distribution shifts in FL. FedConceptEM automatically assigns clients with concept shifts to different models, avoiding the performance drop caused by these shifts. At the same time, clients without concept shifts, even with feature or label skew, are assigned to the same model, improving the robustness of the trained models. Extensive experiments demonstrate that FedConceptEM outperforms other state-of-the-art cluster-based FL methods by a significant margin.
UNO-QA: An Unsupervised Anomaly-Aware Framework with Test-Time Clustering for OCTA Image Quality Assessment
Dec 20, 2022



Abstract:Medical image quality assessment (MIQA) is a vital prerequisite in various medical image analysis applications. Most existing MIQA algorithms are fully supervised that request a large amount of annotated data. However, annotating medical images is time-consuming and labor-intensive. In this paper, we propose an unsupervised anomaly-aware framework with test-time clustering for optical coherence tomography angiography (OCTA) image quality assessment in a setting wherein only a set of high-quality samples are accessible in the training phase. Specifically, a feature-embedding-based low-quality representation module is proposed to quantify the quality of OCTA images and then to discriminate between outstanding quality and non-outstanding quality. Within the non-outstanding quality class, to further distinguish gradable images from ungradable ones, we perform dimension reduction and clustering of multi-scale image features extracted by the trained OCTA quality representation network. Extensive experiments are conducted on one publicly accessible dataset sOCTA-3*3-10k, with superiority of our proposed framework being successfully established.
YoloCurvSeg: You Only Label One Noisy Skeleton for Vessel-style Curvilinear Structure Segmentation
Dec 11, 2022Abstract:Weakly-supervised learning (WSL) has been proposed to alleviate the conflict between data annotation cost and model performance through employing sparsely-grained (i.e., point-, box-, scribble-wise) supervision and has shown promising performance, particularly in the image segmentation field. However, it is still a very challenging problem due to the limited supervision, especially when only a small number of labeled samples are available. Additionally, almost all existing WSL segmentation methods are designed for star-convex structures which are very different from curvilinear structures such as vessels and nerves. In this paper, we propose a novel sparsely annotated segmentation framework for curvilinear structures, named YoloCurvSeg, based on image synthesis. A background generator delivers image backgrounds that closely match real distributions through inpainting dilated skeletons. The extracted backgrounds are then combined with randomly emulated curves generated by a Space Colonization Algorithm-based foreground generator and through a multilayer patch-wise contrastive learning synthesizer. In this way, a synthetic dataset with both images and curve segmentation labels is obtained, at the cost of only one or a few noisy skeleton annotations. Finally, a segmenter is trained with the generated dataset and possibly an unlabeled dataset. The proposed YoloCurvSeg is evaluated on four publicly available datasets (OCTA500, CORN, DRIVE and CHASEDB1) and the results show that YoloCurvSeg outperforms state-of-the-art WSL segmentation methods by large margins. With only one noisy skeleton annotation (respectively 0.14%, 0.02%, 1.4%, and 0.65% of the full annotation), YoloCurvSeg achieves more than 97% of the fully-supervised performance on each dataset. Code and datasets will be released at https://github.com/llmir/YoloCurvSeg.
Diversity Boosted Learning for Domain Generalization with Large Number of Domains
Jul 28, 2022

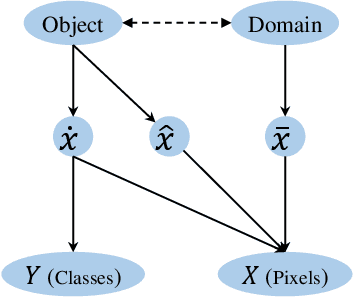
Abstract:Machine learning algorithms minimizing the average training loss usually suffer from poor generalization performance due to the greedy exploitation of correlations among the training data, which are not stable under distributional shifts. It inspires various works for domain generalization (DG), where a series of methods, such as Causal Matching and FISH, work by pairwise domain operations. They would need $O(n^2)$ pairwise domain operations with $n$ domains, where each one is often highly expensive. Moreover, while a common objective in the DG literature is to learn invariant representations against domain-induced spurious correlations, we highlight the importance of mitigating spurious correlations caused by objects. Based on the observation that diversity helps mitigate spurious correlations, we propose a Diversity boosted twO-level saMplIng framework (DOMI) utilizing Determinantal Point Processes (DPPs) to efficiently sample the most informative ones among large number of domains. We show that DOMI helps train robust models against spurious correlations from both domain-side and object-side, substantially enhancing the performance of the backbone DG algorithms on rotated MNIST, rotated Fashion MNIST, and iwildcam datasets.
AADG: Automatic Augmentation for Domain Generalization on Retinal Image Segmentation
Jul 27, 2022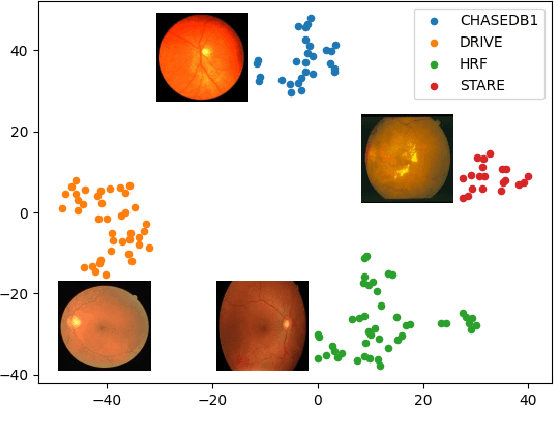
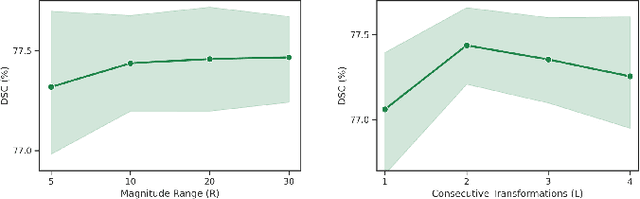
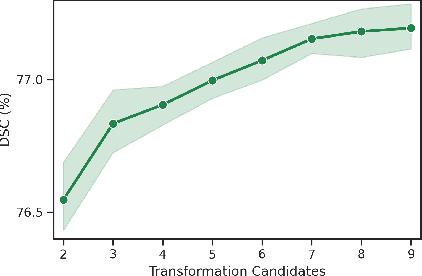
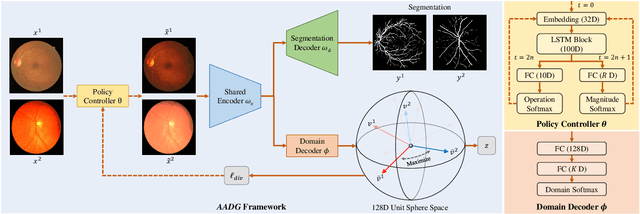
Abstract:Convolutional neural networks have been widely applied to medical image segmentation and have achieved considerable performance. However, the performance may be significantly affected by the domain gap between training data (source domain) and testing data (target domain). To address this issue, we propose a data manipulation based domain generalization method, called Automated Augmentation for Domain Generalization (AADG). Our AADG framework can effectively sample data augmentation policies that generate novel domains and diversify the training set from an appropriate search space. Specifically, we introduce a novel proxy task maximizing the diversity among multiple augmented novel domains as measured by the Sinkhorn distance in a unit sphere space, making automated augmentation tractable. Adversarial training and deep reinforcement learning are employed to efficiently search the objectives. Quantitative and qualitative experiments on 11 publicly-accessible fundus image datasets (four for retinal vessel segmentation, four for optic disc and cup (OD/OC) segmentation and three for retinal lesion segmentation) are comprehensively performed. Two OCTA datasets for retinal vasculature segmentation are further involved to validate cross-modality generalization. Our proposed AADG exhibits state-of-the-art generalization performance and outperforms existing approaches by considerable margins on retinal vessel, OD/OC and lesion segmentation tasks. The learned policies are empirically validated to be model-agnostic and can transfer well to other models. The source code is available at https://github.com/CRazorback/AADG.
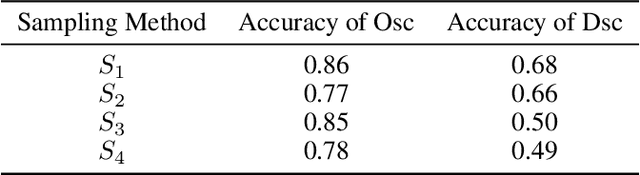
Client Selection in Nonconvex Federated Learning: Improved Convergence Analysis for Optimal Unbiased Sampling Strategy
May 27, 2022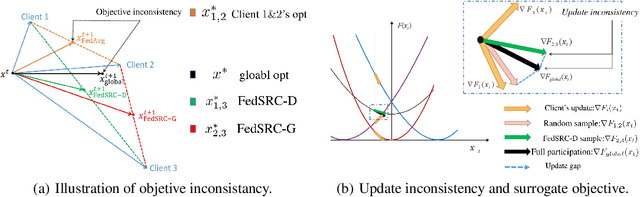
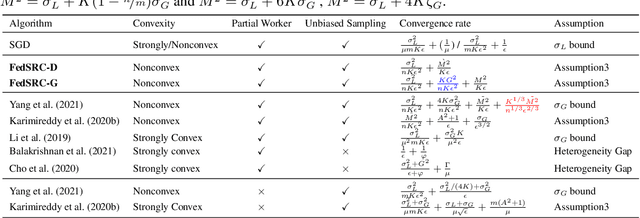
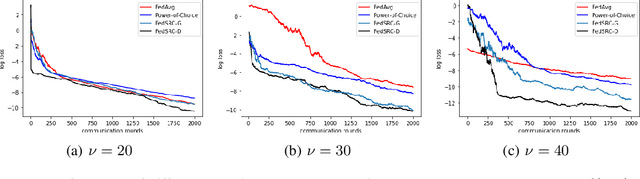
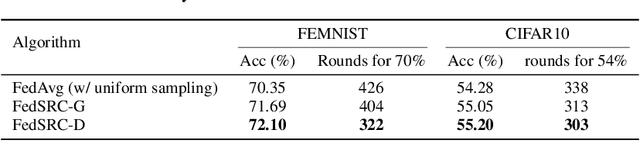
Abstract:Federated learning (FL) is a distributed machine learning paradigm that selects a subset of clients to participate in training to reduce communication burdens. However, partial client participation in FL causes \emph{objective inconsistency}, which can hinder the convergence, while this objective inconsistency has not been analyzed in existing studies on sampling methods. To tackle this issue, we propose an improved analysis method that focuses on the convergence behavior of the practical participated client's objective. Moreover, based on our convergence analysis, we give a novel unbiased sampling strategy, i.e., FedSRC-D, whose sampling probability is proportional to the client's gradient diversity and local variance. FedSRC-D is provable the optimal unbiased sampling in non-convex settings for non-IID FL with respect to the given bounds. Specifically, FedSRC-D achieves $\mathop{O}(\frac{G^2}{\epsilon^2}+\frac{1}{\epsilon^{2/3}})$ higher than SOTA convergence rate of FedAvg, and $\mathop{O}(\frac{G^2}{\epsilon^2})$ higher than other unbiased sampling methods. We corroborate our results with experiments on both synthetic and real data sets.
FedAug: Reducing the Local Learning Bias Improves Federated Learning on Heterogeneous Data
May 27, 2022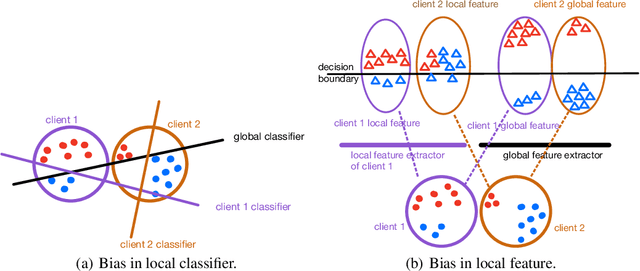
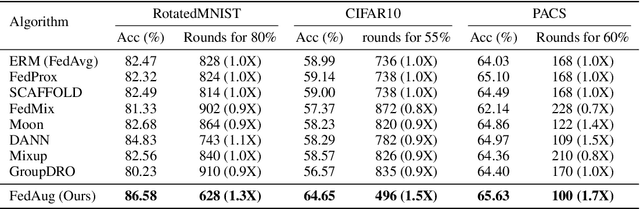
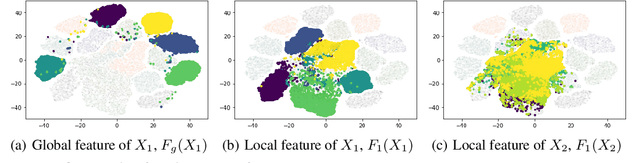
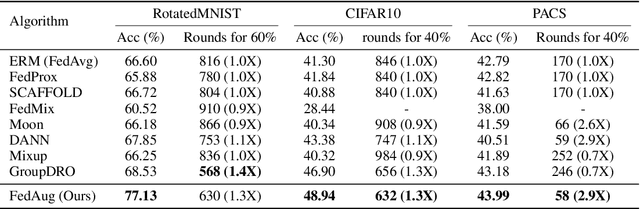
Abstract:Federated Learning (FL) is a machine learning paradigm that learns from data kept locally to safeguard the privacy of clients, whereas local SGD is typically employed on the clients' devices to improve communication efficiency. However, such a scheme is currently constrained by the slow and unstable convergence induced by clients' heterogeneous data. In this work, we identify three under-explored phenomena of the biased local learning that may explain these challenges caused by local updates in supervised FL. As a remedy, we propose FedAug, a novel unified algorithm that reduces the local learning bias on features and classifiers to tackle these challenges. FedAug consists of two components: AugMean and AugCA. AugMean alleviates the bias in the local classifiers by balancing the output distribution of models. AugCA learns client invariant features that are close to global features but considerably distinct from those learned from other input distributions. In a series of experiments, we show that FedAug consistently outperforms other SOTA FL and domain generalization (DG) baselines, in which both two components (i.e., AugMean and AugCA) have individual performance gains.
DS3-Net: Difficulty-perceived Common-to-T1ce Semi-Supervised Multimodal MRI Synthesis Network
Mar 14, 2022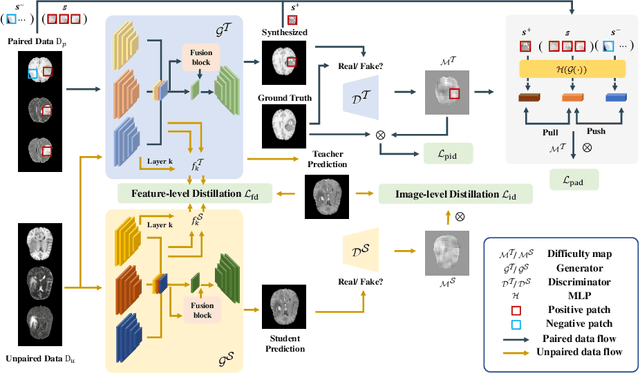
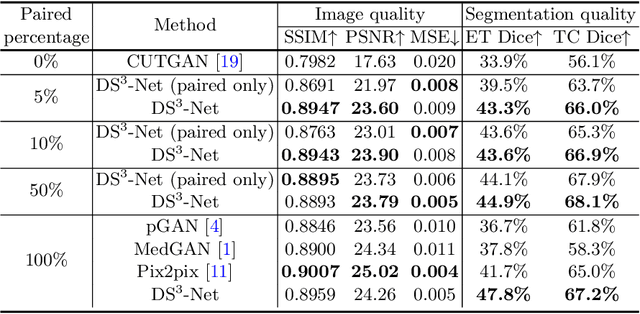
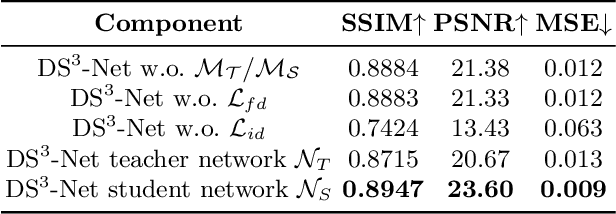
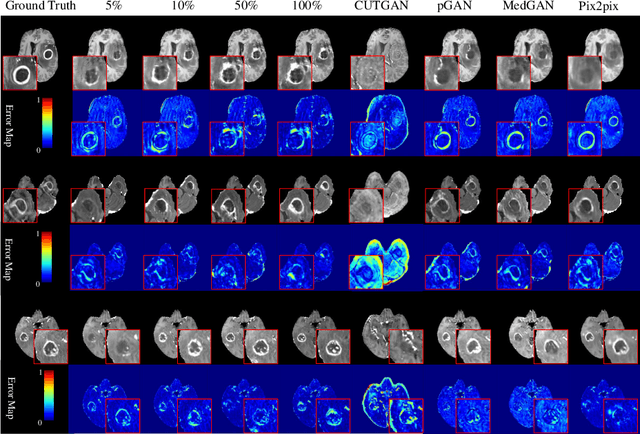
Abstract:Contrast-enhanced T1 (T1ce) is one of the most essential magnetic resonance imaging (MRI) modalities for diagnosing and analyzing brain tumors, especially gliomas. In clinical practice, common MRI modalities such as T1, T2, and fluid attenuation inversion recovery are relatively easy to access while T1ce is more challenging considering the additional cost and potential risk of allergies to the contrast agent. Therefore, it is of great clinical necessity to develop a method to synthesize T1ce from other common modalities. Current paired image translation methods typically have the issue of requiring a large amount of paired data and do not focus on specific regions of interest, e.g., the tumor region, in the synthesization process. To address these issues, we propose a Difficulty-perceived common-to-T1ce Semi-Supervised multimodal MRI Synthesis network (DS3-Net), involving both paired and unpaired data together with dual-level knowledge distillation. DS3-Net predicts a difficulty map to progressively promote the synthesis task. Specifically, a pixelwise constraint and a patchwise contrastive constraint are guided by the predicted difficulty map. Through extensive experiments on the publiclyavailable BraTS2020 dataset, DS3-Net outperforms its supervised counterpart in each respect. Furthermore, with only 5% paired data, the proposed DS3-Net achieves competitive performance with state-of-theart image translation methods utilizing 100% paired data, delivering an average SSIM of 0.8947 and an average PSNR of 23.60.
LesionPaste: One-Shot Anomaly Detection for Medical Images
Mar 12, 2022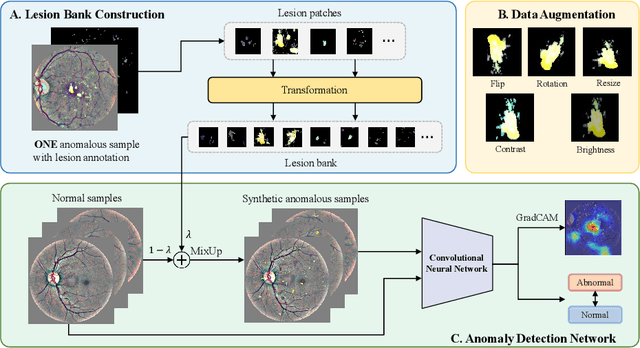
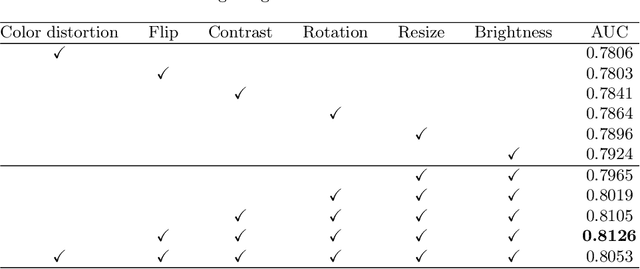
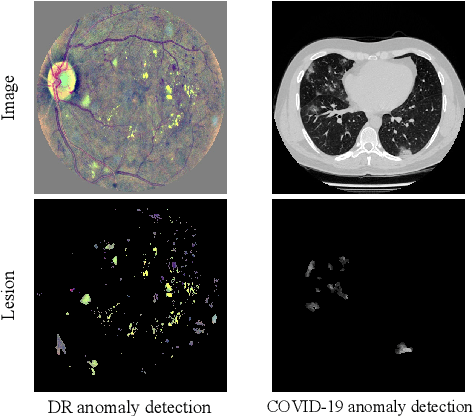
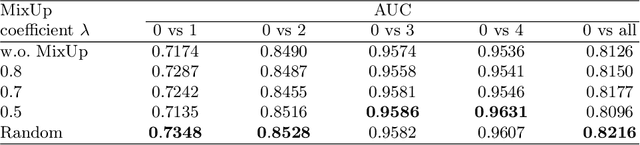
Abstract:Due to the high cost of manually annotating medical images, especially for large-scale datasets, anomaly detection has been explored through training models with only normal data. Lacking prior knowledge of true anomalies is the main reason for the limited application of previous anomaly detection methods, especially in the medical image analysis realm. In this work, we propose a one-shot anomaly detection framework, namely LesionPaste, that utilizes true anomalies from a single annotated sample and synthesizes artificial anomalous samples for anomaly detection. First, a lesion bank is constructed by applying augmentation to randomly selected lesion patches. Then, MixUp is adopted to paste patches from the lesion bank at random positions in normal images to synthesize anomalous samples for training. Finally, a classification network is trained using the synthetic abnormal samples and the true normal data. Extensive experiments are conducted on two publicly-available medical image datasets with different types of abnormalities. On both datasets, our proposed LesionPaste largely outperforms several state-of-the-art unsupervised and semi-supervised anomaly detection methods, and is on a par with the fully-supervised counterpart. To note, LesionPaste is even better than the fully-supervised method in detecting early-stage diabetic retinopathy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge