Shunxing Bao
All-in-SAM: from Weak Annotation to Pixel-wise Nuclei Segmentation with Prompt-based Finetuning
Jul 01, 2023Abstract:The Segment Anything Model (SAM) is a recently proposed prompt-based segmentation model in a generic zero-shot segmentation approach. With the zero-shot segmentation capacity, SAM achieved impressive flexibility and precision on various segmentation tasks. However, the current pipeline requires manual prompts during the inference stage, which is still resource intensive for biomedical image segmentation. In this paper, instead of using prompts during the inference stage, we introduce a pipeline that utilizes the SAM, called all-in-SAM, through the entire AI development workflow (from annotation generation to model finetuning) without requiring manual prompts during the inference stage. Specifically, SAM is first employed to generate pixel-level annotations from weak prompts (e.g., points, bounding box). Then, the pixel-level annotations are used to finetune the SAM segmentation model rather than training from scratch. Our experimental results reveal two key findings: 1) the proposed pipeline surpasses the state-of-the-art (SOTA) methods in a nuclei segmentation task on the public Monuseg dataset, and 2) the utilization of weak and few annotations for SAM finetuning achieves competitive performance compared to using strong pixel-wise annotated data.
Multi-Contrast Computed Tomography Atlas of Healthy Pancreas
Jun 02, 2023Abstract:With the substantial diversity in population demographics, such as differences in age and body composition, the volumetric morphology of pancreas varies greatly, resulting in distinctive variations in shape and appearance. Such variations increase the difficulty at generalizing population-wide pancreas features. A volumetric spatial reference is needed to adapt the morphological variability for organ-specific analysis. Here, we proposed a high-resolution computed tomography (CT) atlas framework specifically optimized for the pancreas organ across multi-contrast CT. We introduce a deep learning-based pre-processing technique to extract the abdominal region of interests (ROIs) and leverage a hierarchical registration pipeline to align the pancreas anatomy across populations. Briefly, DEEDs affine and non-rigid registration are performed to transfer patient abdominal volumes to a fixed high-resolution atlas template. To generate and evaluate the pancreas atlas template, multi-contrast modality CT scans of 443 subjects (without reported history of pancreatic disease, age: 15-50 years old) are processed. Comparing with different registration state-of-the-art tools, the combination of DEEDs affine and non-rigid registration achieves the best performance for the pancreas label transfer across all contrast phases. We further perform external evaluation with another research cohort of 100 de-identified portal venous scans with 13 organs labeled, having the best label transfer performance of 0.504 Dice score in unsupervised setting. The qualitative representation (e.g., average mapping) of each phase creates a clear boundary of pancreas and its distinctive contrast appearance. The deformation surface renderings across scales (e.g., small to large volume) further illustrate the generalizability of the proposed atlas template.
Exploring shared memory architectures for end-to-end gigapixel deep learning
Apr 24, 2023

Abstract:Deep learning has made great strides in medical imaging, enabled by hardware advances in GPUs. One major constraint for the development of new models has been the saturation of GPU memory resources during training. This is especially true in computational pathology, where images regularly contain more than 1 billion pixels. These pathological images are traditionally divided into small patches to enable deep learning due to hardware limitations. In this work, we explore whether the shared GPU/CPU memory architecture on the M1 Ultra systems-on-a-chip (SoCs) recently released by Apple, Inc. may provide a solution. These affordable systems (less than \$5000) provide access to 128 GB of unified memory (Mac Studio with M1 Ultra SoC). As a proof of concept for gigapixel deep learning, we identified tissue from background on gigapixel areas from whole slide images (WSIs). The model was a modified U-Net (4492 parameters) leveraging large kernels and high stride. The M1 Ultra SoC was able to train the model directly on gigapixel images (16000$\times$64000 pixels, 1.024 billion pixels) with a batch size of 1 using over 100 GB of unified memory for the process at an average speed of 1 minute and 21 seconds per batch with Tensorflow 2/Keras. As expected, the model converged with a high Dice score of 0.989 $\pm$ 0.005. Training up until this point took 111 hours and 24 minutes over 4940 steps. Other high RAM GPUs like the NVIDIA A100 (largest commercially accessible at 80 GB, $\sim$\$15000) are not yet widely available (in preview for select regions on Amazon Web Services at \$40.96/hour as a group of 8). This study is a promising step towards WSI-wise end-to-end deep learning with prevalent network architectures.
Segment Anything Model (SAM) for Digital Pathology: Assess Zero-shot Segmentation on Whole Slide Imaging
Apr 09, 2023Abstract:The segment anything model (SAM) was released as a foundation model for image segmentation. The promptable segmentation model was trained by over 1 billion masks on 11M licensed and privacy-respecting images. The model supports zero-shot image segmentation with various segmentation prompts (e.g., points, boxes, masks). It makes the SAM attractive for medical image analysis, especially for digital pathology where the training data are rare. In this study, we evaluate the zero-shot segmentation performance of SAM model on representative segmentation tasks on whole slide imaging (WSI), including (1) tumor segmentation, (2) non-tumor tissue segmentation, (3) cell nuclei segmentation. Core Results: The results suggest that the zero-shot SAM model achieves remarkable segmentation performance for large connected objects. However, it does not consistently achieve satisfying performance for dense instance object segmentation, even with 20 prompts (clicks/boxes) on each image. We also summarized the identified limitations for digital pathology: (1) image resolution, (2) multiple scales, (3) prompt selection, and (4) model fine-tuning. In the future, the few-shot fine-tuning with images from downstream pathological segmentation tasks might help the model to achieve better performance in dense object segmentation.
Cross-scale Multi-instance Learning for Pathological Image Diagnosis
Apr 01, 2023



Abstract:Analyzing high resolution whole slide images (WSIs) with regard to information across multiple scales poses a significant challenge in digital pathology. Multi-instance learning (MIL) is a common solution for working with high resolution images by classifying bags of objects (i.e. sets of smaller image patches). However, such processing is typically performed at a single scale (e.g., 20x magnification) of WSIs, disregarding the vital inter-scale information that is key to diagnoses by human pathologists. In this study, we propose a novel cross-scale MIL algorithm to explicitly aggregate inter-scale relationships into a single MIL network for pathological image diagnosis. The contribution of this paper is three-fold: (1) A novel cross-scale MIL (CS-MIL) algorithm that integrates the multi-scale information and the inter-scale relationships is proposed; (2) A toy dataset with scale-specific morphological features is created and released to examine and visualize differential cross-scale attention; (3) Superior performance on both in-house and public datasets is demonstrated by our simple cross-scale MIL strategy. The official implementation is publicly available at https://github.com/hrlblab/CS-MIL.
Scaling Up 3D Kernels with Bayesian Frequency Re-parameterization for Medical Image Segmentation
Mar 10, 2023Abstract:With the inspiration of vision transformers, the concept of depth-wise convolution revisits to provide a large Effective Receptive Field (ERF) using Large Kernel (LK) sizes for medical image segmentation. However, the segmentation performance might be saturated and even degraded as the kernel sizes scaled up (e.g., $21\times 21\times 21$) in a Convolutional Neural Network (CNN). We hypothesize that convolution with LK sizes is limited to maintain an optimal convergence for locality learning. While Structural Re-parameterization (SR) enhances the local convergence with small kernels in parallel, optimal small kernel branches may hinder the computational efficiency for training. In this work, we propose RepUX-Net, a pure CNN architecture with a simple large kernel block design, which competes favorably with current network state-of-the-art (SOTA) (e.g., 3D UX-Net, SwinUNETR) using 6 challenging public datasets. We derive an equivalency between kernel re-parameterization and the branch-wise variation in kernel convergence. Inspired by the spatial frequency in the human visual system, we extend to vary the kernel convergence into element-wise setting and model the spatial frequency as a Bayesian prior to re-parameterize convolutional weights during training. Specifically, a reciprocal function is leveraged to estimate a frequency-weighted value, which rescales the corresponding kernel element for stochastic gradient descent. From the experimental results, RepUX-Net consistently outperforms 3D SOTA benchmarks with internal validation (FLARE: 0.929 to 0.944), external validation (MSD: 0.901 to 0.932, KiTS: 0.815 to 0.847, LiTS: 0.933 to 0.949, TCIA: 0.736 to 0.779) and transfer learning (AMOS: 0.880 to 0.911) scenarios in Dice Score.
Single Slice Thigh CT Muscle Group Segmentation with Domain Adaptation and Self-Training
Nov 30, 2022Abstract:Objective: Thigh muscle group segmentation is important for assessment of muscle anatomy, metabolic disease and aging. Many efforts have been put into quantifying muscle tissues with magnetic resonance (MR) imaging including manual annotation of individual muscles. However, leveraging publicly available annotations in MR images to achieve muscle group segmentation on single slice computed tomography (CT) thigh images is challenging. Method: We propose an unsupervised domain adaptation pipeline with self-training to transfer labels from 3D MR to single CT slice. First, we transform the image appearance from MR to CT with CycleGAN and feed the synthesized CT images to a segmenter simultaneously. Single CT slices are divided into hard and easy cohorts based on the entropy of pseudo labels inferenced by the segmenter. After refining easy cohort pseudo labels based on anatomical assumption, self-training with easy and hard splits is applied to fine tune the segmenter. Results: On 152 withheld single CT thigh images, the proposed pipeline achieved a mean Dice of 0.888(0.041) across all muscle groups including sartorius, hamstrings, quadriceps femoris and gracilis. muscles Conclusion: To our best knowledge, this is the first pipeline to achieve thigh imaging domain adaptation from MR to CT. The proposed pipeline is effective and robust in extracting muscle groups on 2D single slice CT thigh images.The container is available for public use at https://github.com/MASILab/DA_CT_muscle_seg
Adaptive Contrastive Learning with Dynamic Correlation for Multi-Phase Organ Segmentation
Oct 16, 2022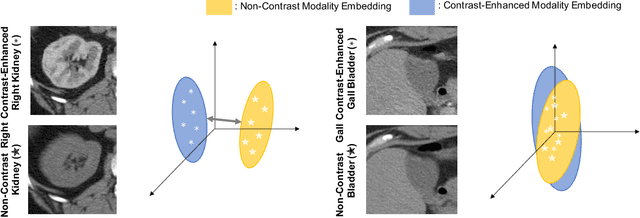
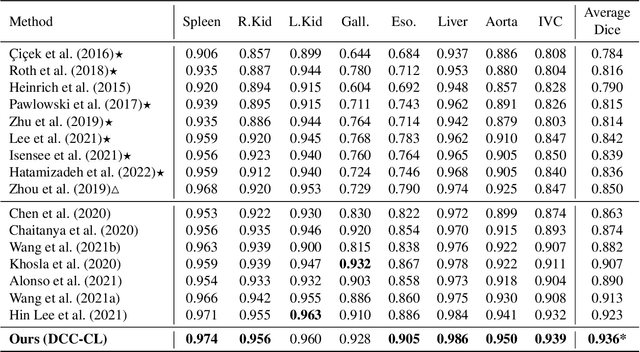
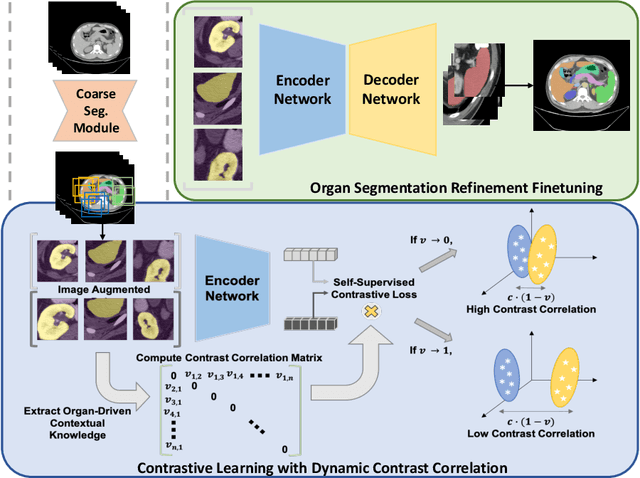
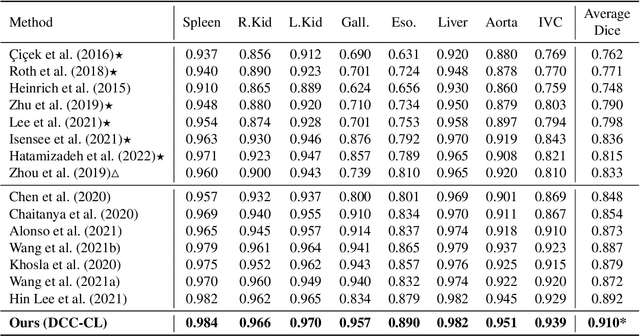
Abstract:Recent studies have demonstrated the superior performance of introducing ``scan-wise" contrast labels into contrastive learning for multi-organ segmentation on multi-phase computed tomography (CT). However, such scan-wise labels are limited: (1) a coarse classification, which could not capture the fine-grained ``organ-wise" contrast variations across all organs; (2) the label (i.e., contrast phase) is typically manually provided, which is error-prone and may introduce manual biases of defining phases. In this paper, we propose a novel data-driven contrastive loss function that adapts the similar/dissimilar contrast relationship between samples in each minibatch at organ-level. Specifically, as variable levels of contrast exist between organs, we hypothesis that the contrast differences in the organ-level can bring additional context for defining representations in the latent space. An organ-wise contrast correlation matrix is computed with mean organ intensities under one-hot attention maps. The goal of adapting the organ-driven correlation matrix is to model variable levels of feature separability at different phases. We evaluate our proposed approach on multi-organ segmentation with both non-contrast CT (NCCT) datasets and the MICCAI 2015 BTCV Challenge contrast-enhance CT (CECT) datasets. Compared to the state-of-the-art approaches, our proposed contrastive loss yields a substantial and significant improvement of 1.41% (from 0.923 to 0.936, p-value$<$0.01) and 2.02% (from 0.891 to 0.910, p-value$<$0.01) on mean Dice scores across all organs with respect to NCCT and CECT cohorts. We further assess the trained model performance with the MICCAI 2021 FLARE Challenge CECT datasets and achieve a substantial improvement of mean Dice score from 0.927 to 0.934 (p-value$<$0.01). The code is available at: https://github.com/MASILab/DCC_CL
3D UX-Net: A Large Kernel Volumetric ConvNet Modernizing Hierarchical Transformer for Medical Image Segmentation
Oct 03, 2022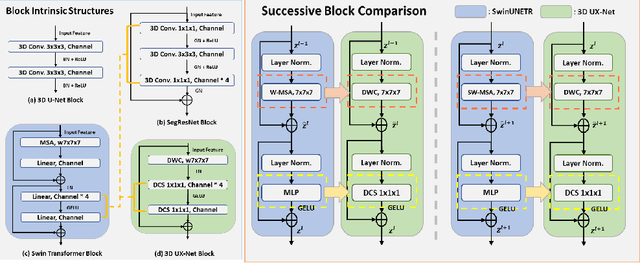
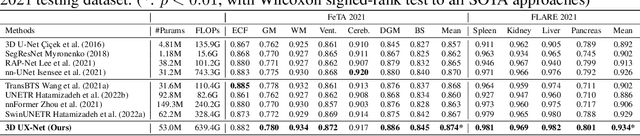
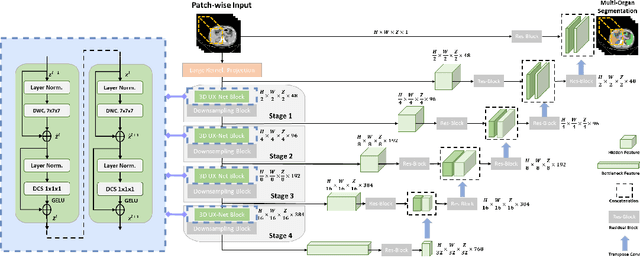
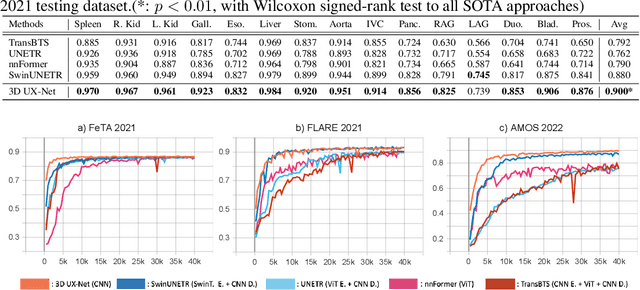
Abstract:Vision transformers (ViTs) have quickly superseded convolutional networks (ConvNets) as the current state-of-the-art (SOTA) models for medical image segmentation. Hierarchical transformers (e.g., Swin Transformers) reintroduced several ConvNet priors and further enhanced the practical viability of adapting volumetric segmentation in 3D medical datasets. The effectiveness of hybrid approaches is largely credited to the large receptive field for non-local self-attention and the large number of model parameters. In this work, we propose a lightweight volumetric ConvNet, termed 3D UX-Net, which adapts the hierarchical transformer using ConvNet modules for robust volumetric segmentation. Specifically, we revisit volumetric depth-wise convolutions with large kernel size (e.g. starting from $7\times7\times7$) to enable the larger global receptive fields, inspired by Swin Transformer. We further substitute the multi-layer perceptron (MLP) in Swin Transformer blocks with pointwise depth convolutions and enhance model performances with fewer normalization and activation layers, thus reducing the number of model parameters. 3D UX-Net competes favorably with current SOTA transformers (e.g. SwinUNETR) using three challenging public datasets on volumetric brain and abdominal imaging: 1) MICCAI Challenge 2021 FLARE, 2) MICCAI Challenge 2021 FeTA, and 3) MICCAI Challenge 2022 AMOS. 3D UX-Net consistently outperforms SwinUNETR with improvement from 0.929 to 0.938 Dice (FLARE2021) and 0.867 to 0.874 Dice (Feta2021). We further evaluate the transfer learning capability of 3D UX-Net with AMOS2022 and demonstrates another improvement of $2.27\%$ Dice (from 0.880 to 0.900). The source code with our proposed model are available at https://github.com/MASILab/3DUX-Net.
Reducing Positional Variance in Cross-sectional Abdominal CT Slices with Deep Conditional Generative Models
Sep 28, 2022Abstract:2D low-dose single-slice abdominal computed tomography (CT) slice enables direct measurements of body composition, which are critical to quantitatively characterizing health relationships on aging. However, longitudinal analysis of body composition changes using 2D abdominal slices is challenging due to positional variance between longitudinal slices acquired in different years. To reduce the positional variance, we extend the conditional generative models to our C-SliceGen that takes an arbitrary axial slice in the abdominal region as the condition and generates a defined vertebral level slice by estimating the structural changes in the latent space. Experiments on 1170 subjects from an in-house dataset and 50 subjects from BTCV MICCAI Challenge 2015 show that our model can generate high quality images in terms of realism and similarity. External experiments on 20 subjects from the Baltimore Longitudinal Study of Aging (BLSA) dataset that contains longitudinal single abdominal slices validate that our method can harmonize the slice positional variance in terms of muscle and visceral fat area. Our approach provides a promising direction of mapping slices from different vertebral levels to a target slice to reduce positional variance for single slice longitudinal analysis. The source code is available at: https://github.com/MASILab/C-SliceGen.
* 11 pages, 4 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge