Mattias P. Heinrich
Adapting the Mean Teacher for keypoint-based lung registration under geometric domain shifts
Jul 01, 2022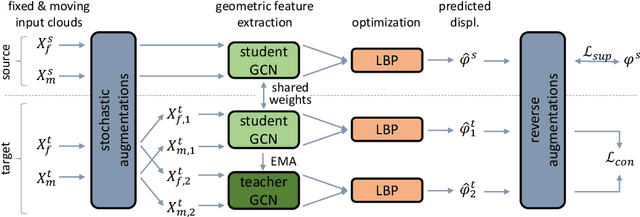
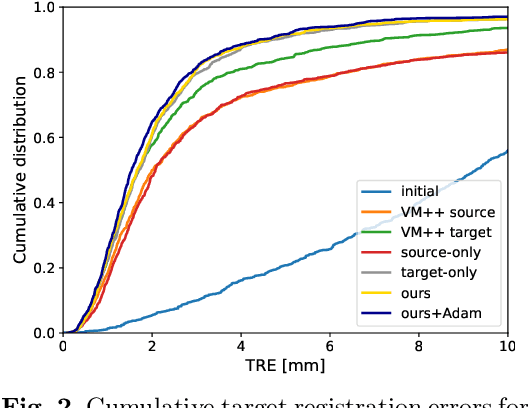
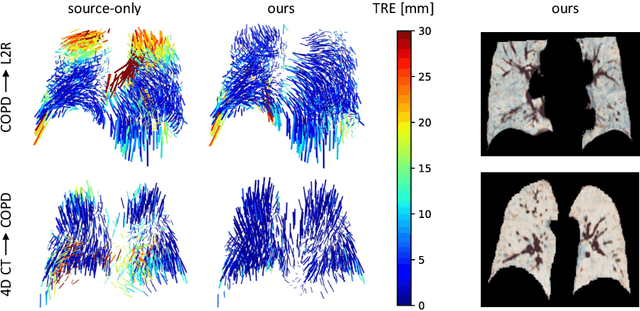
Abstract:Recent deep learning-based methods for medical image registration achieve results that are competitive with conventional optimization algorithms at reduced run times. However, deep neural networks generally require plenty of labeled training data and are vulnerable to domain shifts between training and test data. While typical intensity shifts can be mitigated by keypoint-based registration, these methods still suffer from geometric domain shifts, for instance, due to different fields of view. As a remedy, in this work, we present a novel approach to geometric domain adaptation for image registration, adapting a model from a labeled source to an unlabeled target domain. We build on a keypoint-based registration model, combining graph convolutions for geometric feature learning with loopy belief optimization, and propose to reduce the domain shift through self-ensembling. To this end, we embed the model into the Mean Teacher paradigm. We extend the Mean Teacher to this context by 1) adapting the stochastic augmentation scheme and 2) combining learned feature extraction with differentiable optimization. This enables us to guide the learning process in the unlabeled target domain by enforcing consistent predictions of the learning student and the temporally averaged teacher model. We evaluate the method for exhale-to-inhale lung CT registration under two challenging adaptation scenarios (DIR-Lab 4D CT to COPD, COPD to Learn2Reg). Our method consistently improves on the baseline model by 50%/47% while even matching the accuracy of models trained on target data. Source code is available at https://github.com/multimodallearning/registration-da-mean-teacher.
Voxelmorph++ Going beyond the cranial vault with keypoint supervision and multi-channel instance optimisation
Feb 28, 2022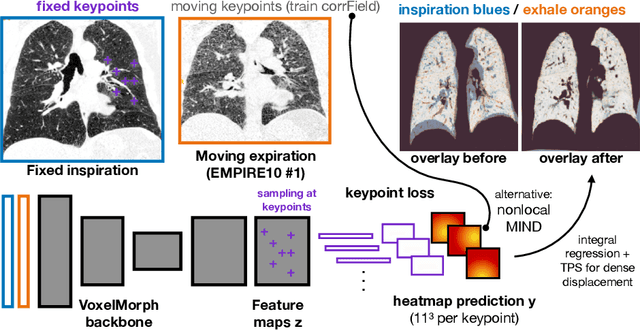
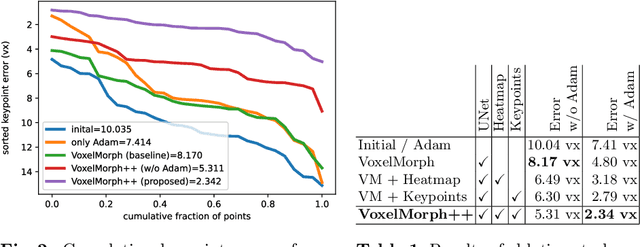
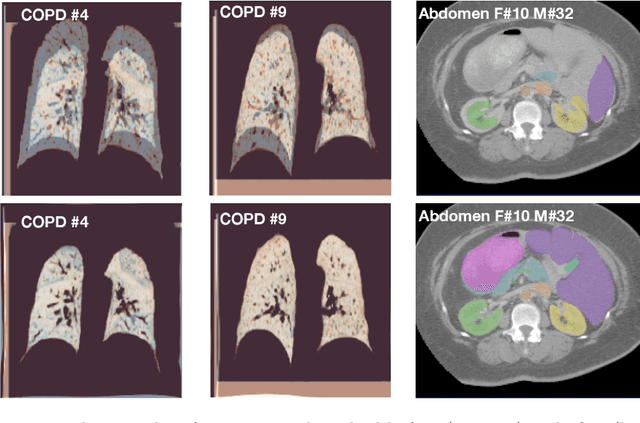
Abstract:The majority of current research in deep learning based image registration addresses inter-patient brain registration with moderate deformation magnitudes. The recent Learn2Reg medical registration benchmark has demonstrated that single-scale U-Net architectures, such as VoxelMorph that directly employ a spatial transformer loss, often do not generalise well beyond the cranial vault and fall short of state-of-the-art performance for abdominal or intra-patient lung registration. Here, we propose two straightforward steps that greatly reduce this gap in accuracy. First, we employ keypoint self-supervision with a novel network head that predicts a discretised heatmap and robustly reduces large deformations for better robustness. Second, we replace multiple learned fine-tuning steps by a single instance optimisation with hand-crafted features and the Adam optimiser. Different to other related work, including FlowNet or PDD-Net, our approach does not require a fully discretised architecture with correlation layer. Our ablation study demonstrates the importance of keypoints in both self-supervised and unsupervised (using only a MIND metric) settings. On a multi-centric inspiration-exhale lung CT dataset, including very challenging COPD scans, our method outperforms VoxelMorph by improving nonlinear alignment by 77% compared to 19% - reaching target registration errors of 2 mm that outperform all but one learning methods published to date. Extending the method to semantic features sets new stat-of-the-art performance on inter-subject abdominal CT registration.
CrossMoDA 2021 challenge: Benchmark of Cross-Modality Domain Adaptation techniques for Vestibular Schwnannoma and Cochlea Segmentation
Jan 08, 2022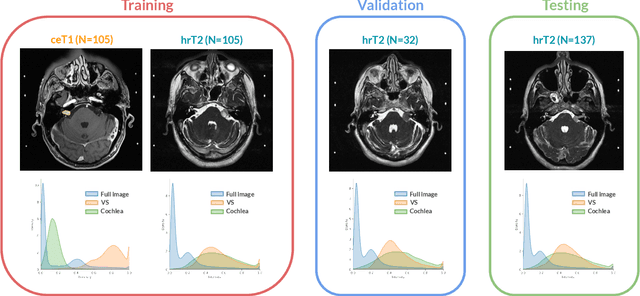
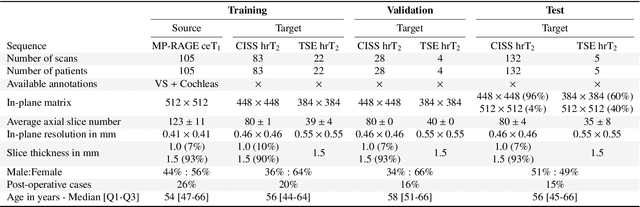
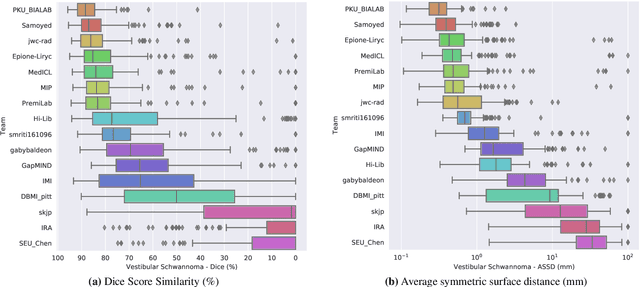
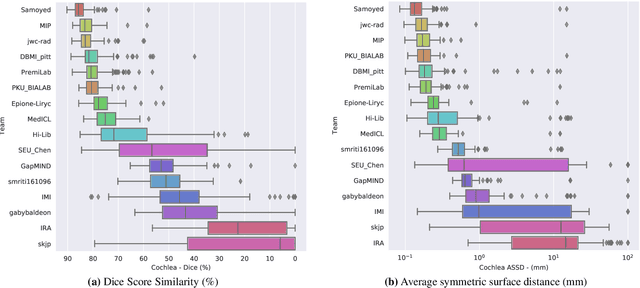
Abstract:Domain Adaptation (DA) has recently raised strong interests in the medical imaging community. While a large variety of DA techniques has been proposed for image segmentation, most of these techniques have been validated either on private datasets or on small publicly available datasets. Moreover, these datasets mostly addressed single-class problems. To tackle these limitations, the Cross-Modality Domain Adaptation (crossMoDA) challenge was organised in conjunction with the 24th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2021). CrossMoDA is the first large and multi-class benchmark for unsupervised cross-modality DA. The challenge's goal is to segment two key brain structures involved in the follow-up and treatment planning of vestibular schwannoma (VS): the VS and the cochleas. Currently, the diagnosis and surveillance in patients with VS are performed using contrast-enhanced T1 (ceT1) MRI. However, there is growing interest in using non-contrast sequences such as high-resolution T2 (hrT2) MRI. Therefore, we created an unsupervised cross-modality segmentation benchmark. The training set provides annotated ceT1 (N=105) and unpaired non-annotated hrT2 (N=105). The aim was to automatically perform unilateral VS and bilateral cochlea segmentation on hrT2 as provided in the testing set (N=137). A total of 16 teams submitted their algorithm for the evaluation phase. The level of performance reached by the top-performing teams is strikingly high (best median Dice - VS:88.4%; Cochleas:85.7%) and close to full supervision (median Dice - VS:92.5%; Cochleas:87.7%). All top-performing methods made use of an image-to-image translation approach to transform the source-domain images into pseudo-target-domain images. A segmentation network was then trained using these generated images and the manual annotations provided for the source image.
Learn2Reg: comprehensive multi-task medical image registration challenge, dataset and evaluation in the era of deep learning
Dec 23, 2021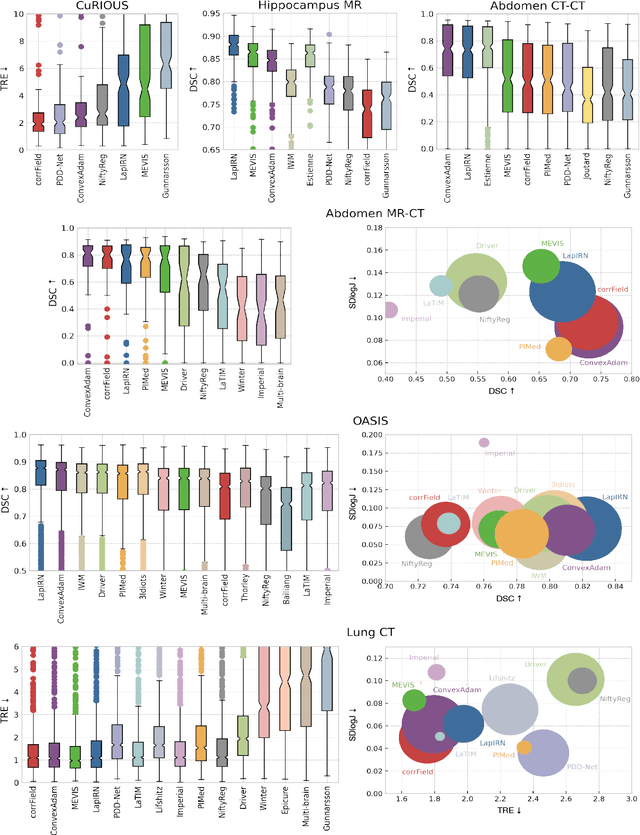
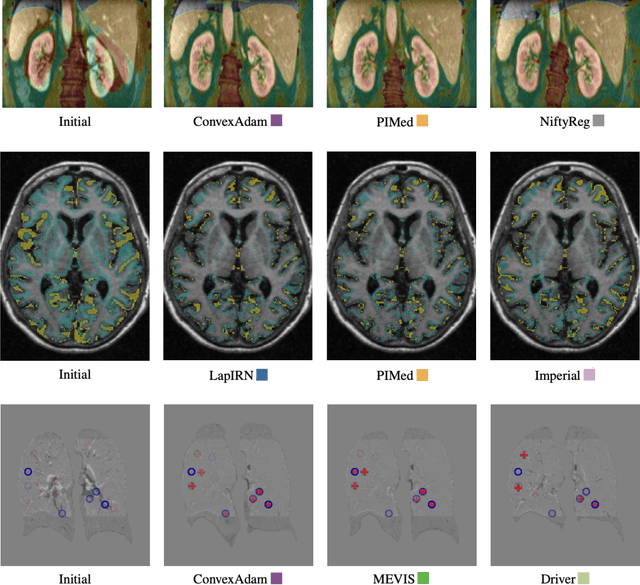
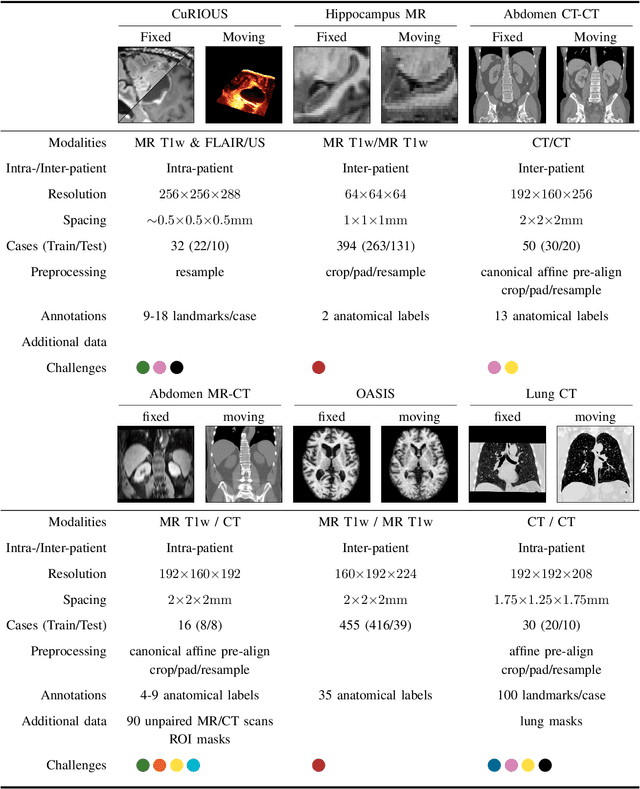
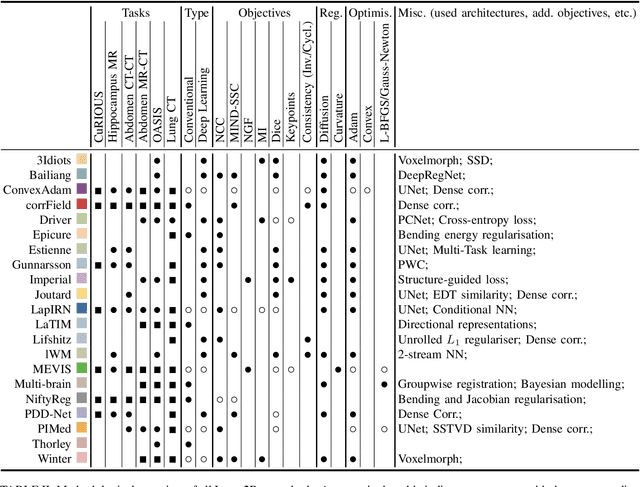
Abstract:Image registration is a fundamental medical image analysis task, and a wide variety of approaches have been proposed. However, only a few studies have comprehensively compared medical image registration approaches on a wide range of clinically relevant tasks, in part because of the lack of availability of such diverse data. This limits the development of registration methods, the adoption of research advances into practice, and a fair benchmark across competing approaches. The Learn2Reg challenge addresses these limitations by providing a multi-task medical image registration benchmark for comprehensive characterisation of deformable registration algorithms. A continuous evaluation will be possible at https://learn2reg.grand-challenge.org. Learn2Reg covers a wide range of anatomies (brain, abdomen, and thorax), modalities (ultrasound, CT, MR), availability of annotations, as well as intra- and inter-patient registration evaluation. We established an easily accessible framework for training and validation of 3D registration methods, which enabled the compilation of results of over 65 individual method submissions from more than 20 unique teams. We used a complementary set of metrics, including robustness, accuracy, plausibility, and runtime, enabling unique insight into the current state-of-the-art of medical image registration. This paper describes datasets, tasks, evaluation methods and results of the challenge, and the results of further analysis of transferability to new datasets, the importance of label supervision, and resulting bias.
Fast 3D registration with accurate optimisation and little learning for Learn2Reg 2021
Dec 06, 2021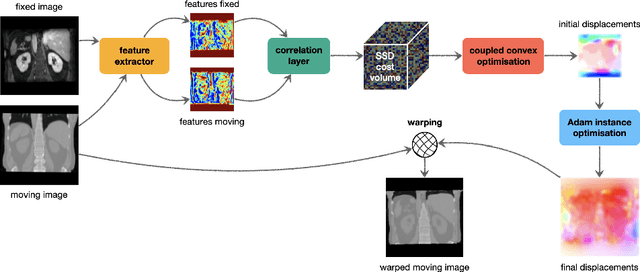
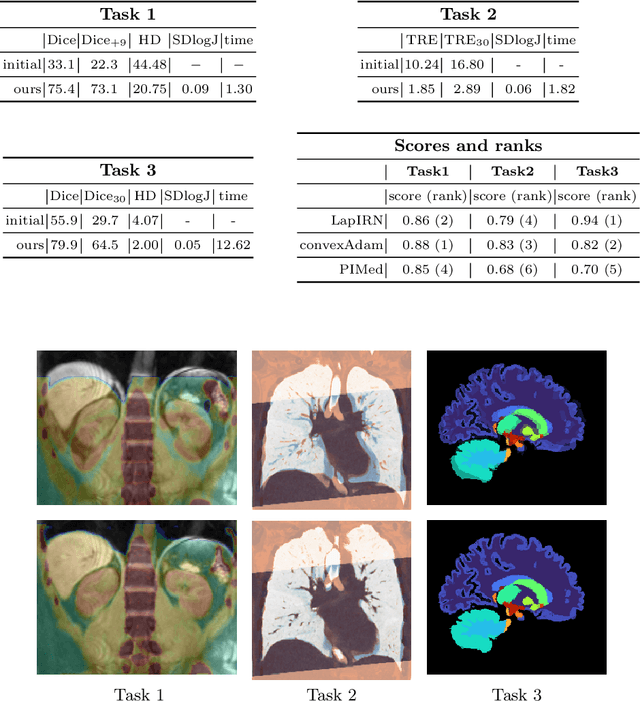
Abstract:Current approaches for deformable medical image registration often struggle to fulfill all of the following criteria: versatile applicability, small computation or training times, and the being able to estimate large deformations. Furthermore, end-to-end networks for supervised training of registration often become overly complex and difficult to train. For the Learn2Reg2021 challenge, we aim to address these issues by decoupling feature learning and geometric alignment. First, we introduce a new very fast and accurate optimisation method. By using discretised displacements and a coupled convex optimisation procedure, we are able to robustly cope with large deformations. With the help of an Adam-based instance optimisation, we achieve very accurate registration performances and by using regularisation, we obtain smooth and plausible deformation fields. Second, to be versatile for different registration tasks, we extract hand-crafted features that are modality and contrast invariant and complement them with semantic features from a task-specific segmentation U-Net. With our results we were able to achieve the overall Learn2Reg2021 challenge's second place, winning Task 1 and being second and third in the other two tasks.
Deep learning based geometric registration for medical images: How accurate can we get without visual features?
Mar 01, 2021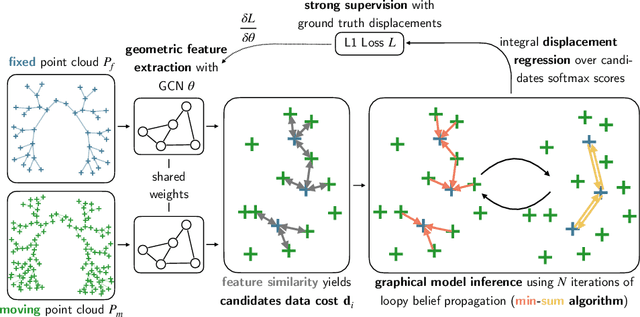
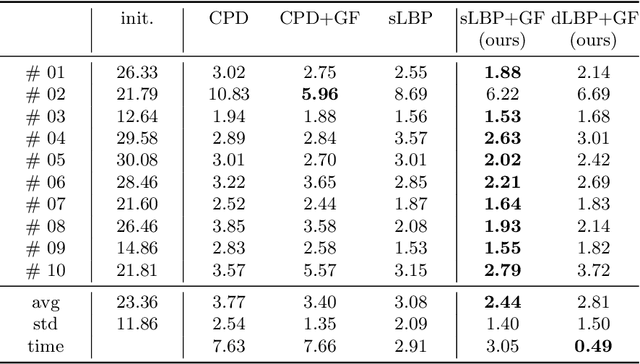
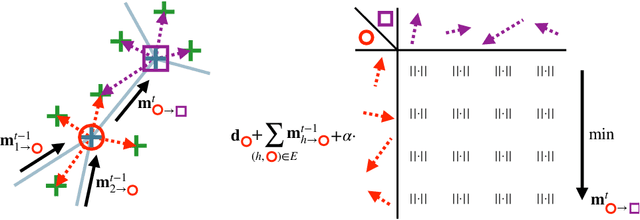
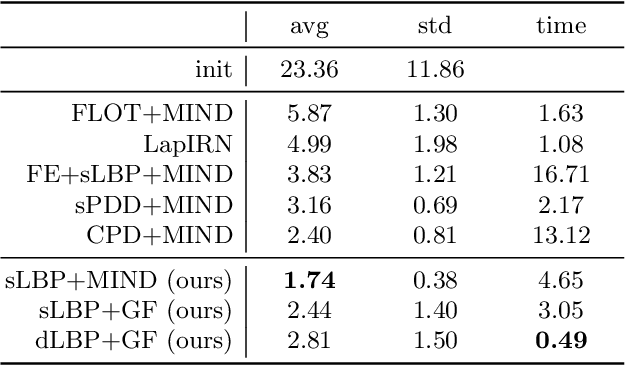
Abstract:As in other areas of medical image analysis, e.g. semantic segmentation, deep learning is currently driving the development of new approaches for image registration. Multi-scale encoder-decoder network architectures achieve state-of-the-art accuracy on tasks such as intra-patient alignment of abdominal CT or brain MRI registration, especially when additional supervision, such as anatomical labels, is available. The success of these methods relies to a large extent on the outstanding ability of deep CNNs to extract descriptive visual features from the input images. In contrast to conventional methods, the explicit inclusion of geometric information plays only a minor role, if at all. In this work we take a look at an exactly opposite approach by investigating a deep learning framework for registration based solely on geometric features and optimisation. We combine graph convolutions with loopy belief message passing to enable highly accurate 3D point cloud registration. Our experimental validation is conducted on complex key-point graphs of inner lung structures, strongly outperforming dense encoder-decoder networks and other point set registration methods. Our code is publicly available at https://github.com/multimodallearning/deep-geo-reg.
Development and Characterization of a Chest CT Atlas
Dec 05, 2020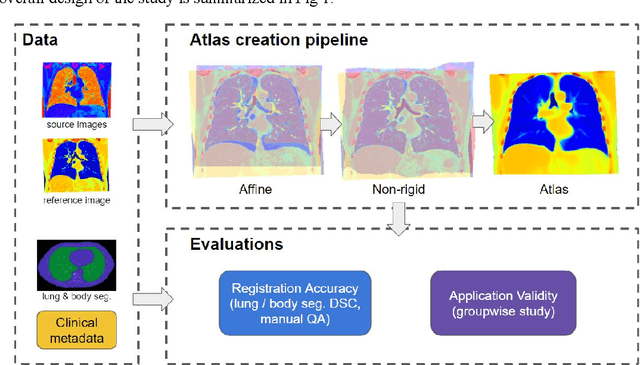
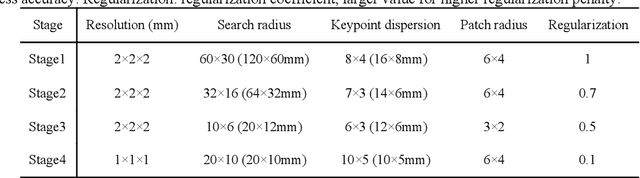
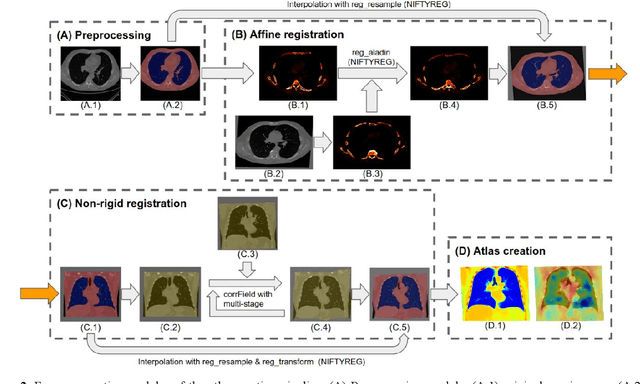
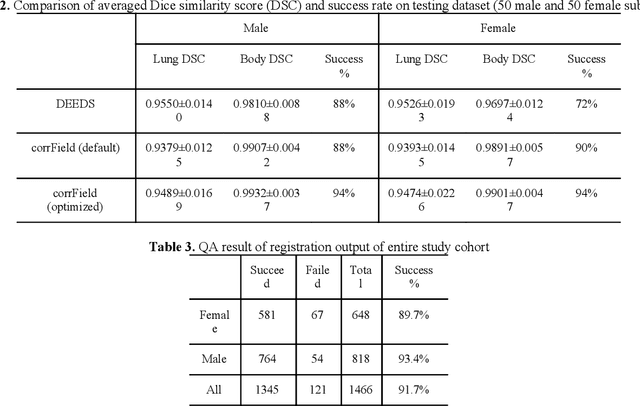
Abstract:A major goal of lung cancer screening is to identify individuals with particular phenotypes that are associated with high risk of cancer. Identifying relevant phenotypes is complicated by the variation in body position and body composition. In the brain, standardized coordinate systems (e.g., atlases) have enabled separate consideration of local features from gross/global structure. To date, no analogous standard atlas has been presented to enable spatial mapping and harmonization in chest computational tomography (CT). In this paper, we propose a thoracic atlas built upon a large low dose CT (LDCT) database of lung cancer screening program. The study cohort includes 466 male and 387 female subjects with no screening detected malignancy (age 46-79 years, mean 64.9 years). To provide spatial mapping, we optimize a multi-stage inter-subject non-rigid registration pipeline for the entire thoracic space. We evaluate the optimized pipeline relative to two baselines with alternative non-rigid registration module: the same software with default parameters and an alternative software. We achieve a significant improvement in terms of registration success rate based on manual QA. For the entire study cohort, the optimized pipeline achieves a registration success rate of 91.7%. The application validity of the developed atlas is evaluated in terms of discriminative capability for different anatomic phenotypes, including body mass index (BMI), chronic obstructive pulmonary disease (COPD), and coronary artery calcification (CAC).
Tackling the Problem of Large Deformations in Deep Learning Based Medical Image Registration Using Displacement Embeddings
May 27, 2020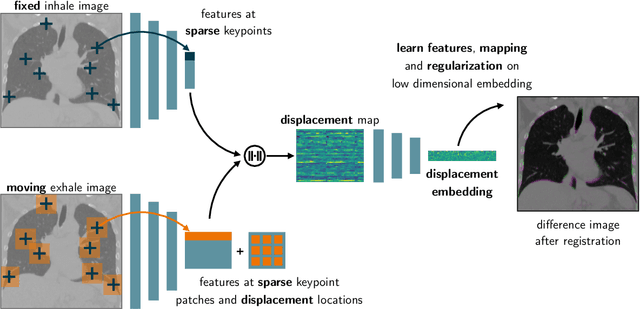
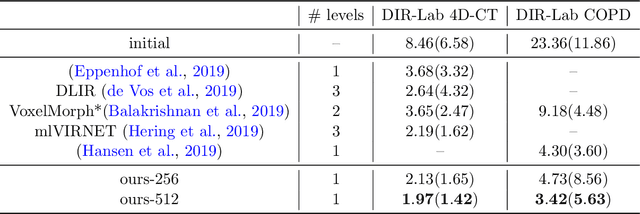
Abstract:Though, deep learning based medical image registration is currently starting to show promising advances, often, it still fells behind conventional frameworks in terms of registration accuracy. This is especially true for applications where large deformations exist, such as registration of interpatient abdominal MRI or inhale-to-exhale CT lung registration. Most current works use U-Net-like architectures to predict dense displacement fields from the input images in different supervised and unsupervised settings. We believe that the U-Net architecture itself to some level limits the ability to predict large deformations (even when using multilevel strategies) and therefore propose a novel approach, where the input images are mapped into a displacement space and final registrations are reconstructed from this embedding. Experiments on inhale-to-exhale CT lung registration demonstrate the ability of our architecture to predict large deformations in a single forward path through our network (leading to errors below 2 mm).
Learning to map between ferns with differentiable binary embedding networks
May 26, 2020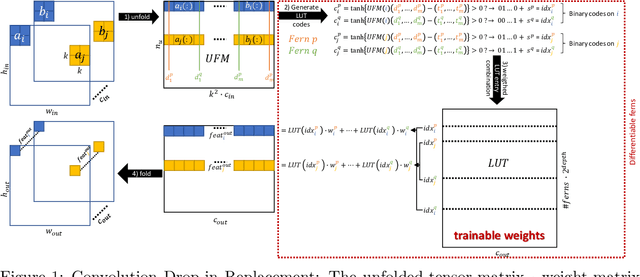
Abstract:Current deep learning methods are based on the repeated, expensive application of convolutions with parameter-intensive weight matrices. In this work, we present a novel concept that enables the application of differentiable random ferns in end-to-end networks. It can then be used as multiplication-free convolutional layer alternative in deep network architectures. Our experiments on the binary classification task of the TUPAC'16 challenge demonstrate improved results over the state-of-the-art binary XNOR net and only slightly worse performance than its 2x more parameter intensive floating point CNN counterpart.
Segmentation of Retinal Low-Cost Optical Coherence Tomography Images using Deep Learning
Jan 23, 2020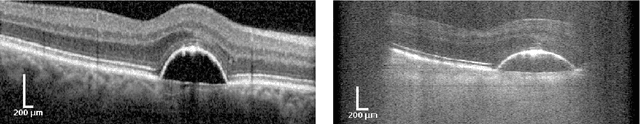
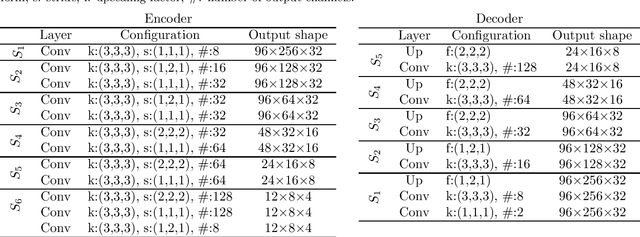
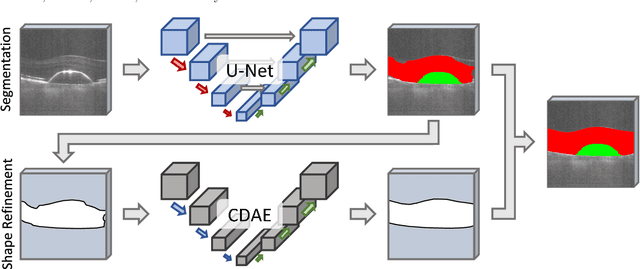
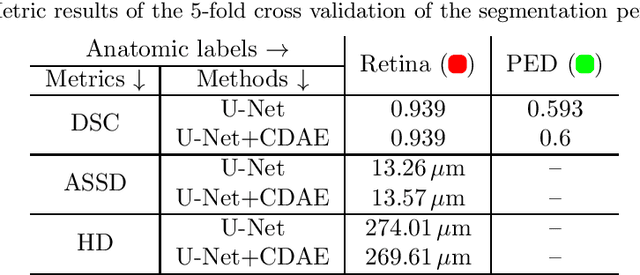
Abstract:The treatment of age-related macular degeneration (AMD) requires continuous eye exams using optical coherence tomography (OCT). The need for treatment is determined by the presence or change of disease-specific OCT-based biomarkers. Therefore, the monitoring frequency has a significant influence on the success of AMD therapy. However, the monitoring frequency of current treatment schemes is not individually adapted to the patient and therefore often insufficient. While a higher monitoring frequency would have a positive effect on the success of treatment, in practice it can only be achieved with a home monitoring solution. One of the key requirements of a home monitoring OCT system is a computer-aided diagnosis to automatically detect and quantify pathological changes using specific OCT-based biomarkers. In this paper, for the first time, retinal scans of a novel self-examination low-cost full-field OCT (SELF-OCT) are segmented using a deep learning-based approach. A convolutional neural network (CNN) is utilized to segment the total retina as well as pigment epithelial detachments (PED). It is shown that the CNN-based approach can segment the retina with high accuracy, whereas the segmentation of the PED proves to be challenging. In addition, a convolutional denoising autoencoder (CDAE) refines the CNN prediction, which has previously learned retinal shape information. It is shown that the CDAE refinement can correct segmentation errors caused by artifacts in the OCT image.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge