Mahmud Mossa-Basha
Unified and Semantically Grounded Domain Adaptation for Medical Image Segmentation
Aug 12, 2025



Abstract:Most prior unsupervised domain adaptation approaches for medical image segmentation are narrowly tailored to either the source-accessible setting, where adaptation is guided by source-target alignment, or the source-free setting, which typically resorts to implicit supervision mechanisms such as pseudo-labeling and model distillation. This substantial divergence in methodological designs between the two settings reveals an inherent flaw: the lack of an explicit, structured construction of anatomical knowledge that naturally generalizes across domains and settings. To bridge this longstanding divide, we introduce a unified, semantically grounded framework that supports both source-accessible and source-free adaptation. Fundamentally distinct from all prior works, our framework's adaptability emerges naturally as a direct consequence of the model architecture, without the need for any handcrafted adaptation strategies. Specifically, our model learns a domain-agnostic probabilistic manifold as a global space of anatomical regularities, mirroring how humans establish visual understanding. Thus, the structural content in each image can be interpreted as a canonical anatomy retrieved from the manifold and a spatial transformation capturing individual-specific geometry. This disentangled, interpretable formulation enables semantically meaningful prediction with intrinsic adaptability. Extensive experiments on challenging cardiac and abdominal datasets show that our framework achieves state-of-the-art results in both settings, with source-free performance closely approaching its source-accessible counterpart, a level of consistency rarely observed in prior works. Beyond quantitative improvement, we demonstrate strong interpretability of the proposed framework via manifold traversal for smooth shape manipulation.
Benchmarking the CoW with the TopCoW Challenge: Topology-Aware Anatomical Segmentation of the Circle of Willis for CTA and MRA
Dec 29, 2023



Abstract:The Circle of Willis (CoW) is an important network of arteries connecting major circulations of the brain. Its vascular architecture is believed to affect the risk, severity, and clinical outcome of serious neuro-vascular diseases. However, characterizing the highly variable CoW anatomy is still a manual and time-consuming expert task. The CoW is usually imaged by two angiographic imaging modalities, magnetic resonance angiography (MRA) and computed tomography angiography (CTA), but there exist limited public datasets with annotations on CoW anatomy, especially for CTA. Therefore we organized the TopCoW Challenge in 2023 with the release of an annotated CoW dataset and invited submissions worldwide for the CoW segmentation task, which attracted over 140 registered participants from four continents. TopCoW dataset was the first public dataset with voxel-level annotations for CoW's 13 vessel components, made possible by virtual-reality (VR) technology. It was also the first dataset with paired MRA and CTA from the same patients. TopCoW challenge aimed to tackle the CoW characterization problem as a multiclass anatomical segmentation task with an emphasis on topological metrics. The top performing teams managed to segment many CoW components to Dice scores around 90%, but with lower scores for communicating arteries and rare variants. There were also topological mistakes for predictions with high Dice scores. Additional topological analysis revealed further areas for improvement in detecting certain CoW components and matching CoW variant's topology accurately. TopCoW represented a first attempt at benchmarking the CoW anatomical segmentation task for MRA and CTA, both morphologically and topologically.
nnDetection for Intracranial Aneurysms Detection and Localization
May 22, 2023Abstract:Intracranial aneurysms are a commonly occurring and life-threatening condition, affecting approximately 3.2% of the general population. Consequently, detecting these aneurysms plays a crucial role in their management. Lesion detection involves the simultaneous localization and categorization of abnormalities within medical images. In this study, we employed the nnDetection framework, a self-configuring framework specifically designed for 3D medical object detection, to detect and localize the 3D coordinates of aneurysms effectively. To capture and extract diverse features associated with aneurysms, we utilized TOF-MRA and structural MRI, both obtained from the ADAM dataset. The performance of our proposed deep learning model was assessed through the utilization of free-response receiver operative characteristics for evaluation purposes. The model's weights and 3D prediction of the bounding box of TOF-MRA are publicly available at https://github.com/orouskhani/AneurysmDetection.
Deep Open Snake Tracker for Vessel Tracing
Jul 19, 2021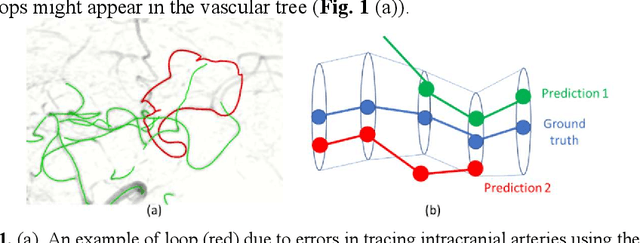
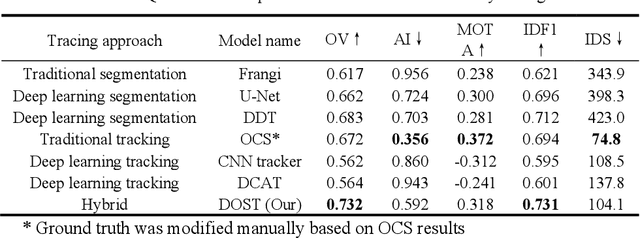
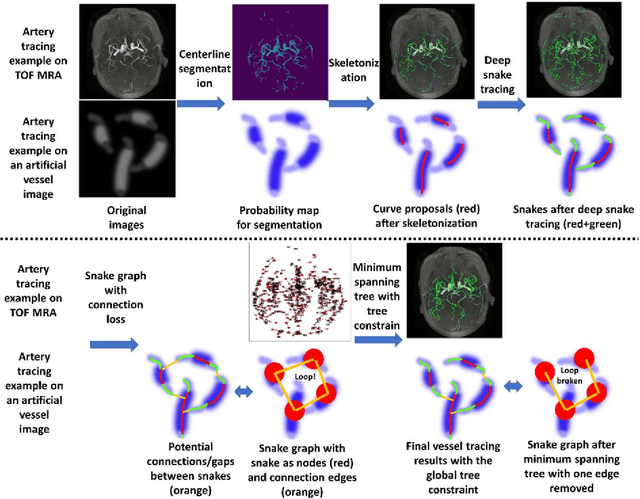
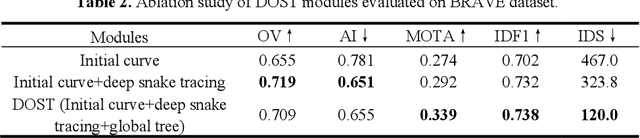
Abstract:Vessel tracing by modeling vascular structures in 3D medical images with centerlines and radii can provide useful information for vascular health. Existing algorithms have been developed but there are certain persistent problems such as incomplete or inaccurate vessel tracing, especially in complicated vascular beds like the intracranial arteries. We propose here a deep learning based open curve active contour model (DOST) to trace vessels in 3D images. Initial curves were proposed from a centerline segmentation neural network. Then data-driven machine knowledge was used to predict the stretching direction and vessel radius of the initial curve, while the active contour model (as human knowledge) maintained smoothness and intensity fitness of curves. Finally, considering the nonloop topology of most vasculatures, individually traced vessels were connected into a tree topology by applying a minimum spanning tree algorithm on a global connection graph. We evaluated DOST on a Time-of-Flight (TOF) MRA intracranial artery dataset and demonstrated its superior performance over existing segmentation-based and tracking-based vessel tracing methods. In addition, DOST showed strong adaptability on different imaging modalities (CTA, MR T1 SPACE) and vascular beds (coronary arteries).
Y-net: 3D intracranial artery segmentation using a convolutional autoencoder
Dec 19, 2017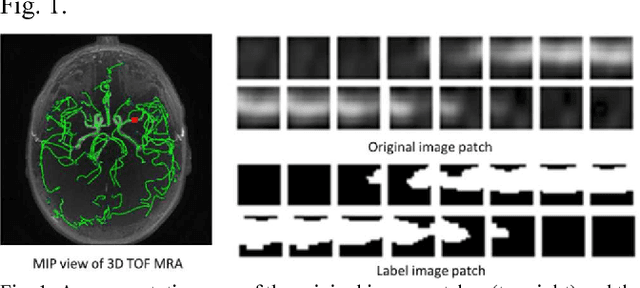
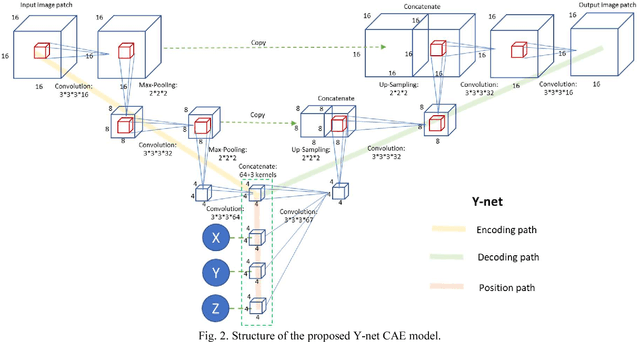
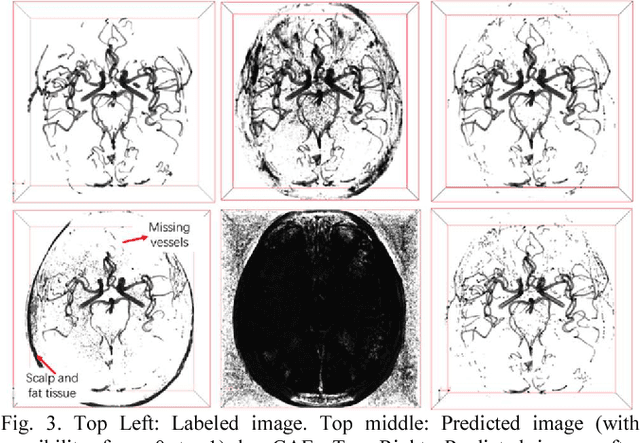
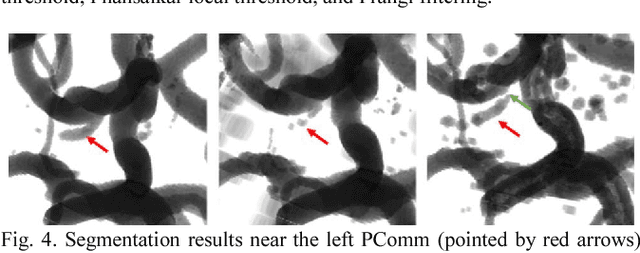
Abstract:Automated segmentation of intracranial arteries on magnetic resonance angiography (MRA) allows for quantification of cerebrovascular features, which provides tools for understanding aging and pathophysiological adaptations of the cerebrovascular system. Using a convolutional autoencoder (CAE) for segmentation is promising as it takes advantage of the autoencoder structure in effective noise reduction and feature extraction by representing high dimensional information with low dimensional latent variables. In this report, an optimized CAE model (Y-net) was trained to learn a 3D segmentation model of intracranial arteries from 49 cases of MRA data. The trained model was shown to perform better than the three traditional segmentation methods in both binary classification and visual evaluation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge