Ling Zhang
Missouri S&T EMC Laboratory, Rolla, MO, USA
Automated Discovery of Real-Time Network Camera Data From Heterogeneous Web Pages
Mar 23, 2021

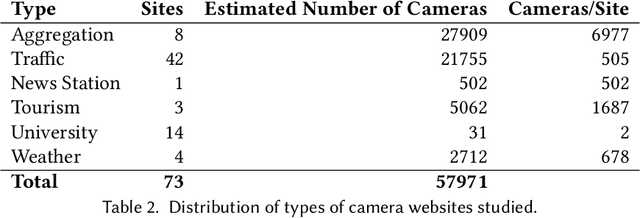
Abstract:Reduction in the cost of Network Cameras along with a rise in connectivity enables entities all around the world to deploy vast arrays of camera networks. Network cameras offer real-time visual data that can be used for studying traffic patterns, emergency response, security, and other applications. Although many sources of Network Camera data are available, collecting the data remains difficult due to variations in programming interface and website structures. Previous solutions rely on manually parsing the target website, taking many hours to complete. We create a general and automated solution for aggregating Network Camera data spread across thousands of uniquely structured web pages. We analyze heterogeneous web page structures and identify common characteristics among 73 sample Network Camera websites (each website has multiple web pages). These characteristics are then used to build an automated camera discovery module that crawls and aggregates Network Camera data. Our system successfully extracts 57,364 Network Cameras from 237,257 unique web pages.
3D Graph Anatomy Geometry-Integrated Network for Pancreatic Mass Segmentation, Diagnosis, and Quantitative Patient Management
Dec 08, 2020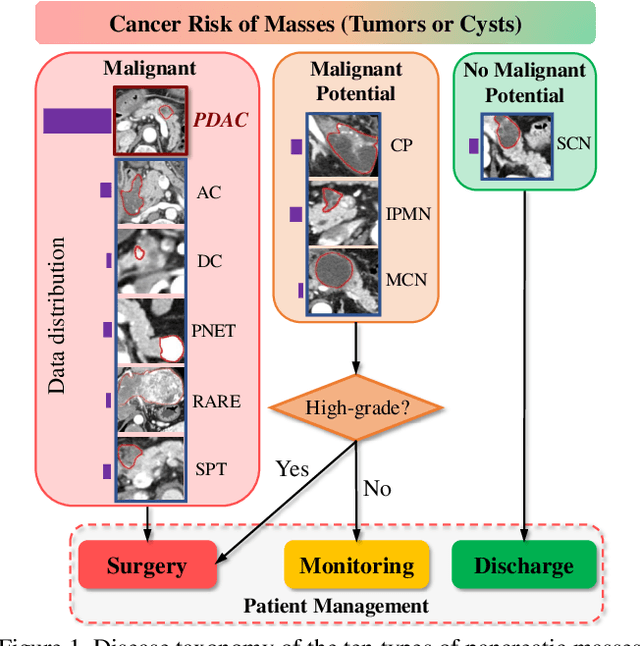
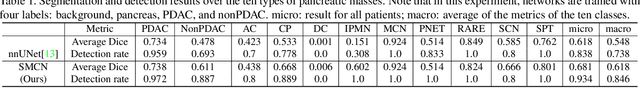
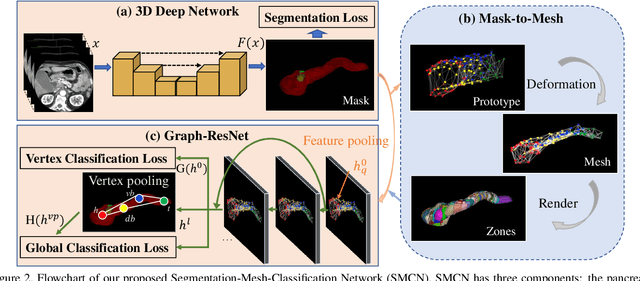
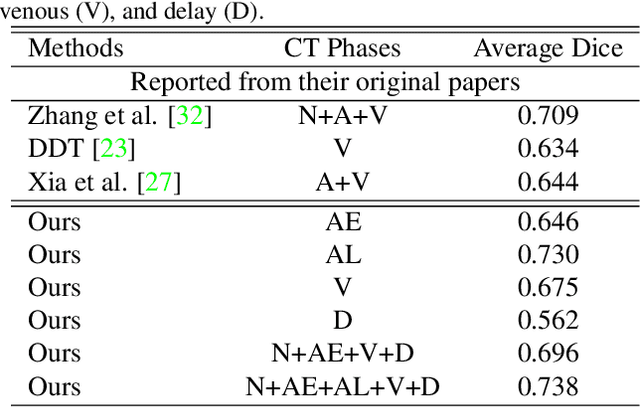
Abstract:The pancreatic disease taxonomy includes ten types of masses (tumors or cysts)[20,8]. Previous work focuses on developing segmentation or classification methods only for certain mass types. Differential diagnosis of all mass types is clinically highly desirable [20] but has not been investigated using an automated image understanding approach. We exploit the feasibility to distinguish pancreatic ductal adenocarcinoma (PDAC) from the nine other nonPDAC masses using multi-phase CT imaging. Both image appearance and the 3D organ-mass geometry relationship are critical. We propose a holistic segmentation-mesh-classification network (SMCN) to provide patient-level diagnosis, by fully utilizing the geometry and location information, which is accomplished by combining the anatomical structure and the semantic detection-by-segmentation network. SMCN learns the pancreas and mass segmentation task and builds an anatomical correspondence-aware organ mesh model by progressively deforming a pancreas prototype on the raw segmentation mask (i.e., mask-to-mesh). A new graph-based residual convolutional network (Graph-ResNet), whose nodes fuse the information of the mesh model and feature vectors extracted from the segmentation network, is developed to produce the patient-level differential classification results. Extensive experiments on 661 patients' CT scans (five phases per patient) show that SMCN can improve the mass segmentation and detection accuracy compared to the strong baseline method nnUNet (e.g., for nonPDAC, Dice: 0.611 vs. 0.478; detection rate: 89% vs. 70%), achieve similar sensitivity and specificity in differentiating PDAC and nonPDAC as expert radiologists (i.e., 94% and 90%), and obtain results comparable to a multimodality test [20] that combines clinical, imaging, and molecular testing for clinical management of patients.
DeepPrognosis: Preoperative Prediction of Pancreatic Cancer Survival and Surgical Margin via Contrast-Enhanced CT Imaging
Aug 26, 2020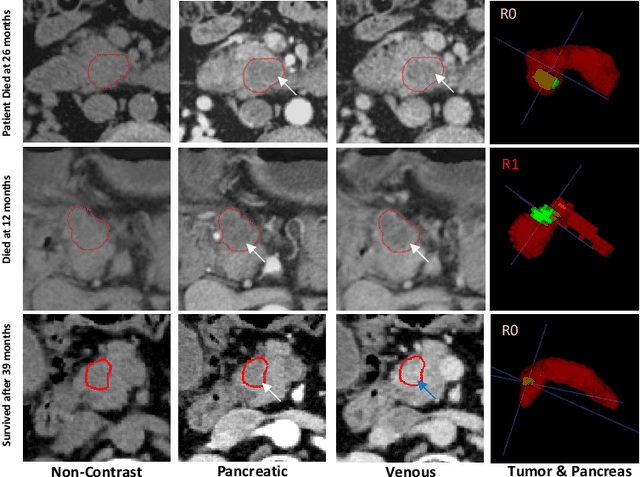
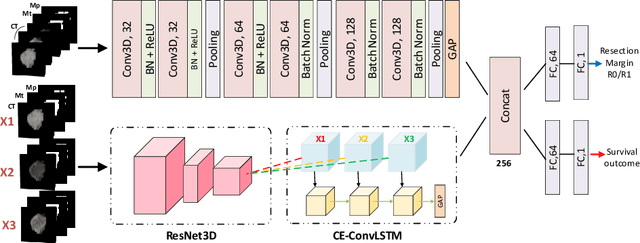
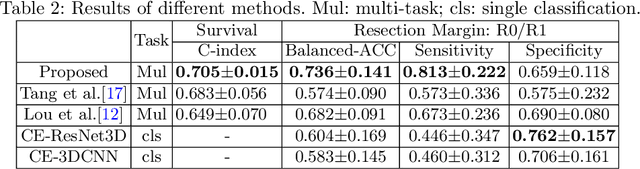
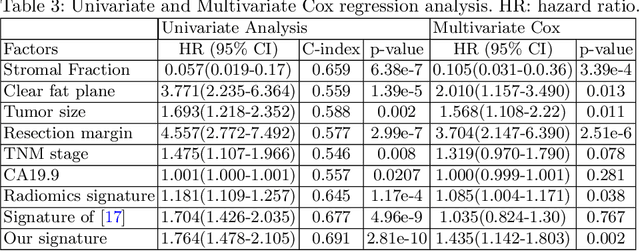
Abstract:Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers and carries a dismal prognosis. Surgery remains the best chance of a potential cure for patients who are eligible for initial resection of PDAC. However, outcomes vary significantly even among the resected patients of the same stage and received similar treatments. Accurate preoperative prognosis of resectable PDACs for personalized treatment is thus highly desired. Nevertheless, there are no automated methods yet to fully exploit the contrast-enhanced computed tomography (CE-CT) imaging for PDAC. Tumor attenuation changes across different CT phases can reflect the tumor internal stromal fractions and vascularization of individual tumors that may impact the clinical outcomes. In this work, we propose a novel deep neural network for the survival prediction of resectable PDAC patients, named as 3D Contrast-Enhanced Convolutional Long Short-Term Memory network(CE-ConvLSTM), which can derive the tumor attenuation signatures or patterns from CE-CT imaging studies. We present a multi-task CNN to accomplish both tasks of outcome and margin prediction where the network benefits from learning the tumor resection margin related features to improve survival prediction. The proposed framework can improve the prediction performances compared with existing state-of-the-art survival analysis approaches. The tumor signature built from our model has evidently added values to be combined with the existing clinical staging system.
Robust Pancreatic Ductal Adenocarcinoma Segmentation with Multi-Institutional Multi-Phase Partially-Annotated CT Scans
Aug 24, 2020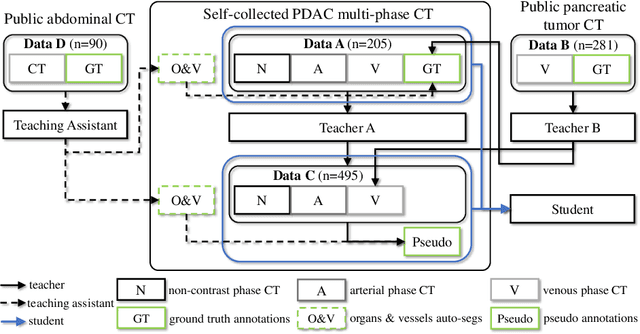
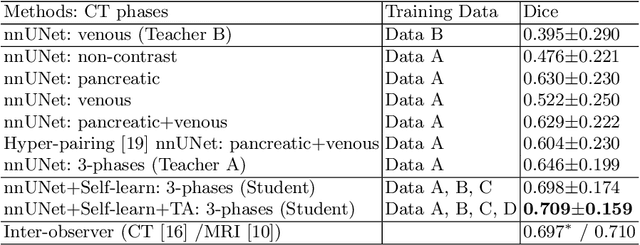
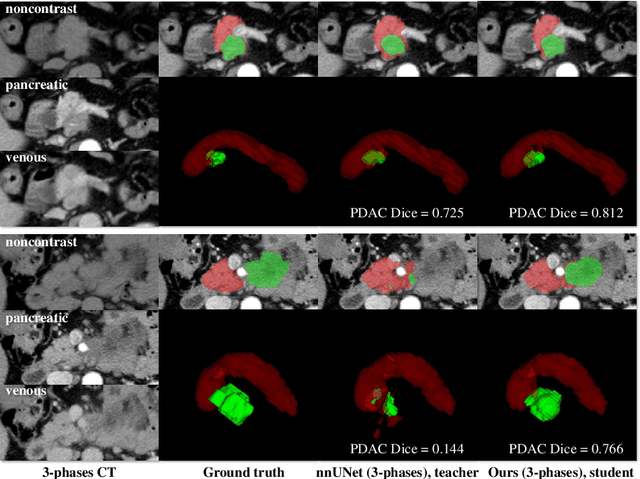
Abstract:Accurate and automated tumor segmentation is highly desired since it has the great potential to increase the efficiency and reproducibility of computing more complete tumor measurements and imaging biomarkers, comparing to (often partial) human measurements. This is probably the only viable means to enable the large-scale clinical oncology patient studies that utilize medical imaging. Deep learning approaches have shown robust segmentation performances for certain types of tumors, e.g., brain tumors in MRI imaging, when a training dataset with plenty of pixel-level fully-annotated tumor images is available. However, more than often, we are facing the challenge that only (very) limited annotations are feasible to acquire, especially for hard tumors. Pancreatic ductal adenocarcinoma (PDAC) segmentation is one of the most challenging tumor segmentation tasks, yet critically important for clinical needs. Previous work on PDAC segmentation is limited to the moderate amounts of annotated patient images (n<300) from venous or venous+arterial phase CT scans. Based on a new self-learning framework, we propose to train the PDAC segmentation model using a much larger quantity of patients (n~=1,000), with a mix of annotated and un-annotated venous or multi-phase CT images. Pseudo annotations are generated by combining two teacher models with different PDAC segmentation specialties on unannotated images, and can be further refined by a teaching assistant model that identifies associated vessels around the pancreas. A student model is trained on both manual and pseudo annotated multi-phase images. Experiment results show that our proposed method provides an absolute improvement of 6.3% Dice score over the strong baseline of nnUNet trained on annotated images, achieving the performance (Dice = 0.71) similar to the inter-observer variability between radiologists.
Searching Learning Strategy with Reinforcement Learning for 3D Medical Image Segmentation
Jun 10, 2020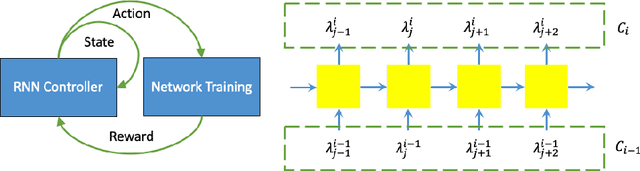
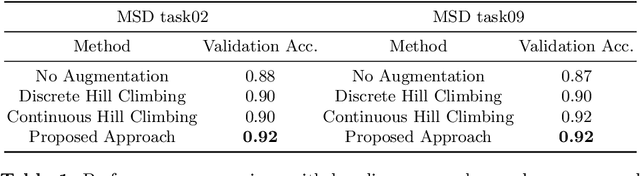
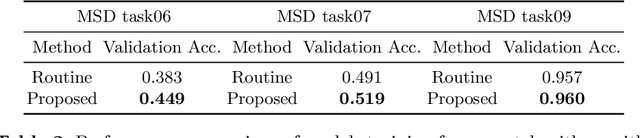
Abstract:Deep neural network (DNN) based approaches have been widely investigated and deployed in medical image analysis. For example, fully convolutional neural networks (FCN) achieve the state-of-the-art performance in several applications of 2D/3D medical image segmentation. Even the baseline neural network models (U-Net, V-Net, etc.) have been proven to be very effective and efficient when the training process is set up properly. Nevertheless, to fully exploit the potentials of neural networks, we propose an automated searching approach for the optimal training strategy with reinforcement learning. The proposed approach can be utilized for tuning hyper-parameters, and selecting necessary data augmentation with certain probabilities. The proposed approach is validated on several tasks of 3D medical image segmentation. The performance of the baseline model is boosted after searching, and it can achieve comparable accuracy to other manually-tuned state-of-the-art segmentation approaches.
* 9 pages, 1 figures
Self-supervised Modal and View Invariant Feature Learning
May 28, 2020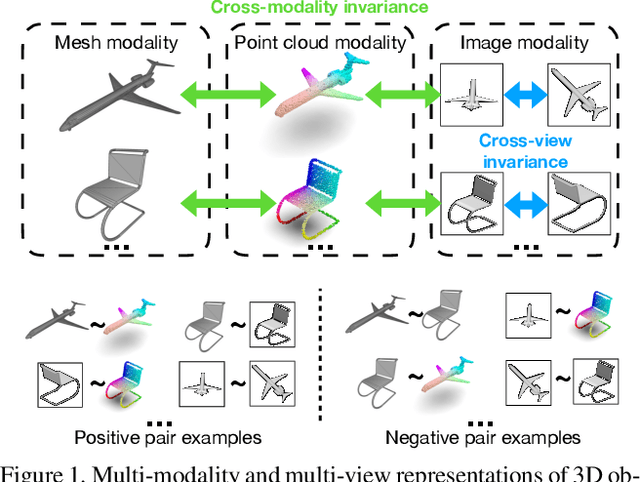
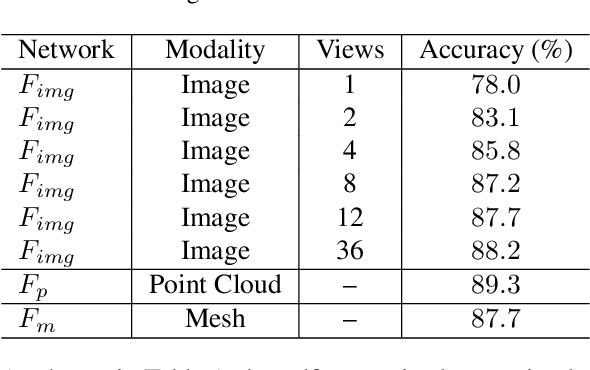
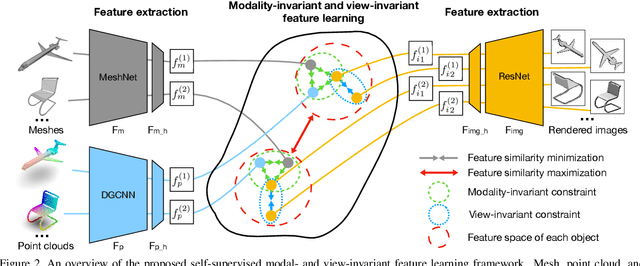
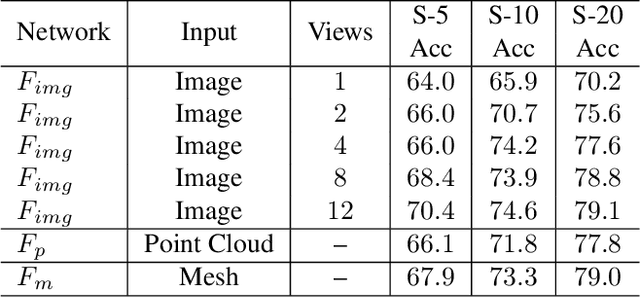
Abstract:Most of the existing self-supervised feature learning methods for 3D data either learn 3D features from point cloud data or from multi-view images. By exploring the inherent multi-modality attributes of 3D objects, in this paper, we propose to jointly learn modal-invariant and view-invariant features from different modalities including image, point cloud, and mesh with heterogeneous networks for 3D data. In order to learn modal- and view-invariant features, we propose two types of constraints: cross-modal invariance constraint and cross-view invariant constraint. Cross-modal invariance constraint forces the network to maximum the agreement of features from different modalities for same objects, while the cross-view invariance constraint forces the network to maximum agreement of features from different views of images for same objects. The quality of learned features has been tested on different downstream tasks with three modalities of data including point cloud, multi-view images, and mesh. Furthermore, the invariance cross different modalities and views are evaluated with the cross-modal retrieval task. Extensive evaluation results demonstrate that the learned features are robust and have strong generalizability across different tasks.
Self-supervised Feature Learning by Cross-modality and Cross-view Correspondences
Apr 13, 2020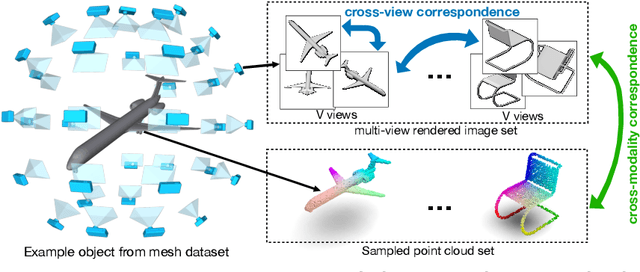
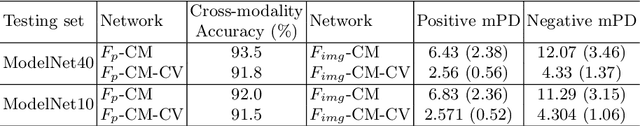
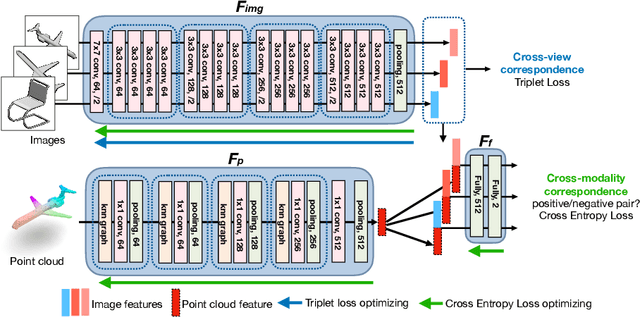
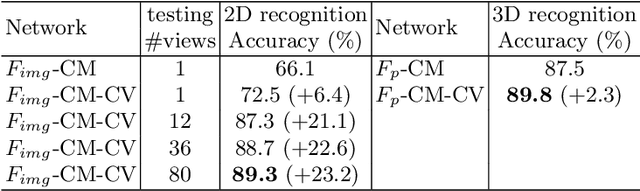
Abstract:The success of supervised learning requires large-scale ground truth labels which are very expensive, time-consuming, or may need special skills to annotate. To address this issue, many self- or un-supervised methods are developed. Unlike most existing self-supervised methods to learn only 2D image features or only 3D point cloud features, this paper presents a novel and effective self-supervised learning approach to jointly learn both 2D image features and 3D point cloud features by exploiting cross-modality and cross-view correspondences without using any human annotated labels. Specifically, 2D image features of rendered images from different views are extracted by a 2D convolutional neural network, and 3D point cloud features are extracted by a graph convolution neural network. Two types of features are fed into a two-layer fully connected neural network to estimate the cross-modality correspondence. The three networks are jointly trained (i.e. cross-modality) by verifying whether two sampled data of different modalities belong to the same object, meanwhile, the 2D convolutional neural network is additionally optimized through minimizing intra-object distance while maximizing inter-object distance of rendered images in different views (i.e. cross-view). The effectiveness of the learned 2D and 3D features is evaluated by transferring them on five different tasks including multi-view 2D shape recognition, 3D shape recognition, multi-view 2D shape retrieval, 3D shape retrieval, and 3D part-segmentation. Extensive evaluations on all the five different tasks across different datasets demonstrate strong generalization and effectiveness of the learned 2D and 3D features by the proposed self-supervised method.
RIS-GAN: Explore Residual and Illumination with Generative Adversarial Networks for Shadow Removal
Dec 24, 2019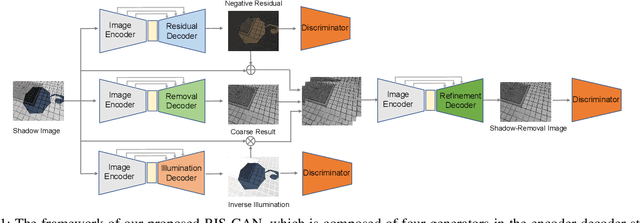
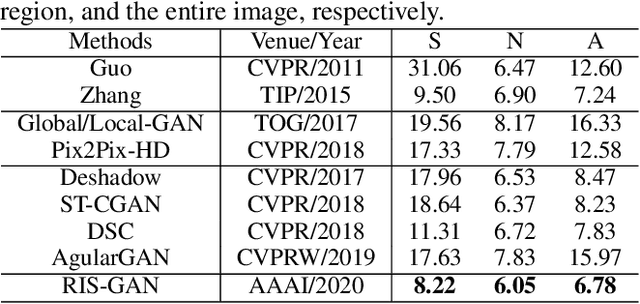

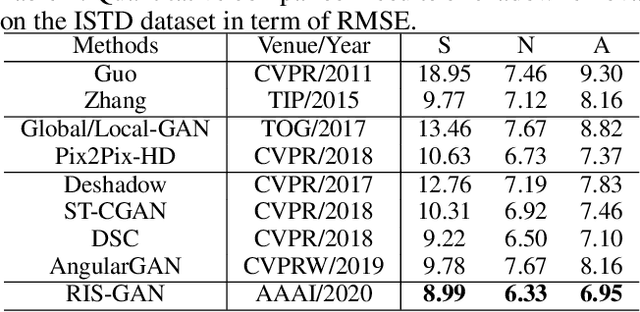
Abstract:Residual images and illumination estimation have been proved very helpful in image enhancement. In this paper, we propose a general and novel framework RIS-GAN which explores residual and illumination with Generative Adversarial Networks for shadow removal. Combined with the coarse shadow-removal image, the estimated negative residual images and inverse illumination maps can be used to generate indirect shadow-removal images to refine the coarse shadow-removal result to the fine shadow-free image in a coarse-to-fine fashion. Three discriminators are designed to distinguish whether the predicted negative residual images, shadow-removal images, and the inverse illumination maps are real or fake jointly compared with the corresponding ground-truth information. To our best knowledge, we are the first one to explore residual and illumination for shadow removal. We evaluate our proposed method on two benchmark datasets, i.e., SRD and ISTD, and the extensive experiments demonstrate that our proposed method achieves the superior performance to state-of-the-arts, although we have no particular shadow-aware components designed in our generators.
Weakly supervised segmentation from extreme points
Oct 02, 2019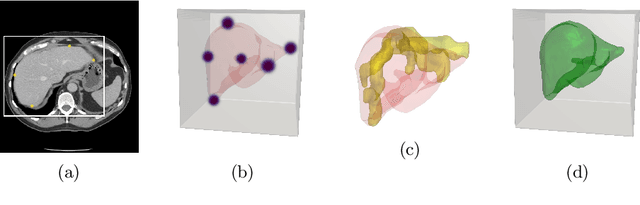
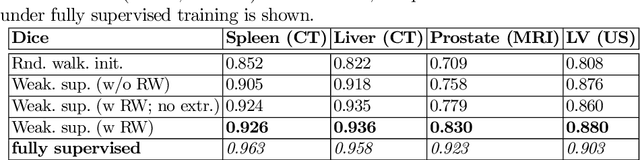
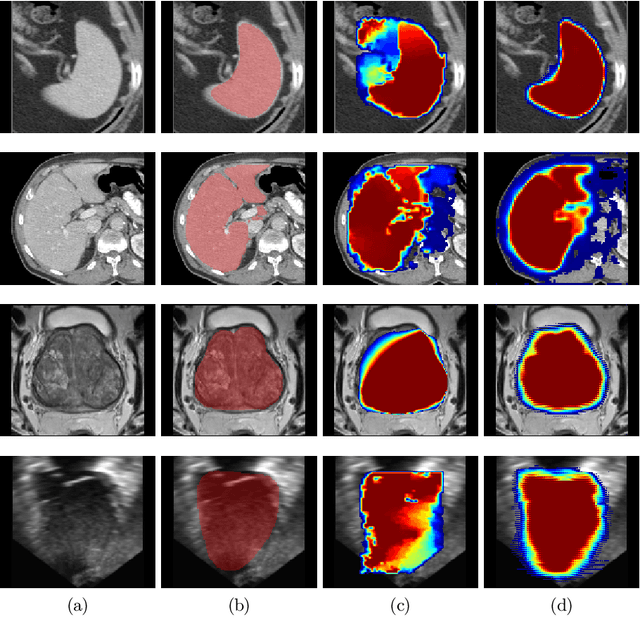
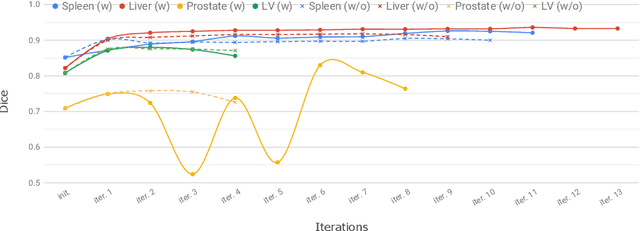
Abstract:Annotation of medical images has been a major bottleneck for the development of accurate and robust machine learning models. Annotation is costly and time-consuming and typically requires expert knowledge, especially in the medical domain. Here, we propose to use minimal user interaction in the form of extreme point clicks in order to train a segmentation model that can, in turn, be used to speed up the annotation of medical images. We use extreme points in each dimension of a 3D medical image to constrain an initial segmentation based on the random walker algorithm. This segmentation is then used as a weak supervisory signal to train a fully convolutional network that can segment the organ of interest based on the provided user clicks. We show that the network's predictions can be refined through several iterations of training and prediction using the same weakly annotated data. Ultimately, our method has the potential to speed up the generation process of new training datasets for the development of new machine learning and deep learning-based models for, but not exclusively, medical image analysis.
ARGAN: Attentive Recurrent Generative Adversarial Network for Shadow Detection and Removal
Aug 04, 2019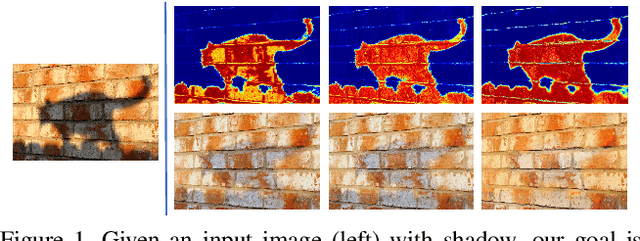
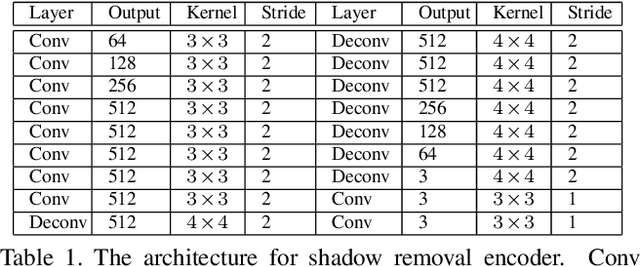
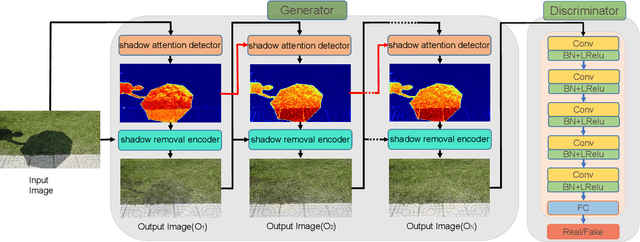
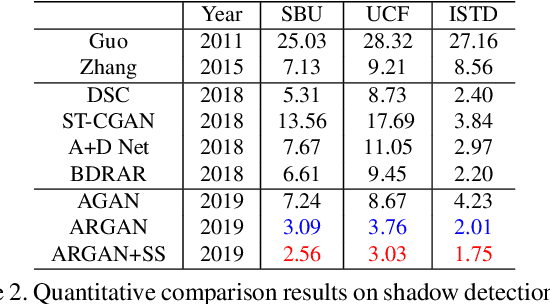
Abstract:In this paper we propose an attentive recurrent generative adversarial network (ARGAN) to detect and remove shadows in an image. The generator consists of multiple progressive steps. At each step a shadow attention detector is firstly exploited to generate an attention map which specifies shadow regions in the input image.Given the attention map, a negative residual by a shadow remover encoder will recover a shadow-lighter or even a shadow-free image. A discriminator is designed to classify whether the output image in the last progressive step is real or fake. Moreover, ARGAN is suitable to be trained with a semi-supervised strategy to make full use of sufficient unsupervised data. The experiments on four public datasets have demonstrated that our ARGAN is robust to detect both simple and complex shadows and to produce more realistic shadow removal results. It outperforms the state-of-the-art methods, especially in detail of recovering shadow areas.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge