Kai Ma
School of Electrical Engineering, Yanshan University, Qinhuangdao, China
Difficulty-aware Glaucoma Classification with Multi-Rater Consensus Modeling
Jul 29, 2020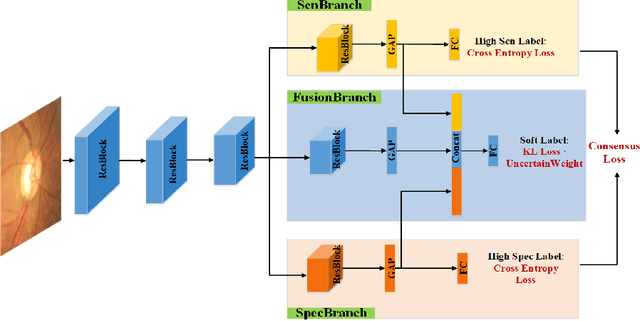
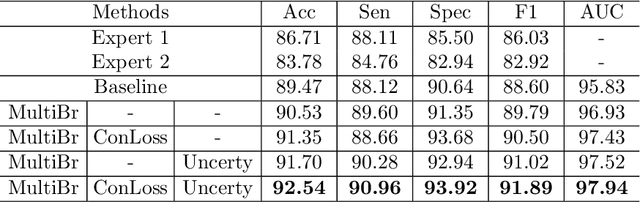
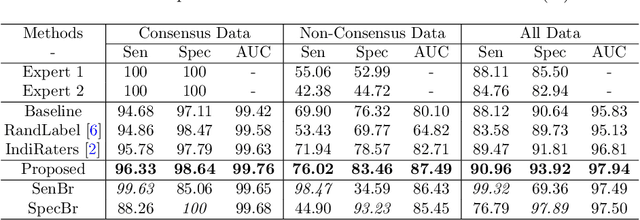
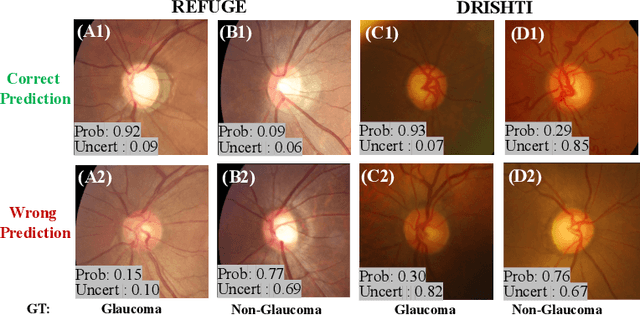
Abstract:Medical images are generally labeled by multiple experts before the final ground-truth labels are determined. Consensus or disagreement among experts regarding individual images reflects the gradeability and difficulty levels of the image. However, when being used for model training, only the final ground-truth label is utilized, while the critical information contained in the raw multi-rater gradings regarding the image being an easy/hard case is discarded. In this paper, we aim to take advantage of the raw multi-rater gradings to improve the deep learning model performance for the glaucoma classification task. Specifically, a multi-branch model structure is proposed to predict the most sensitive, most specifical and a balanced fused result for the input images. In order to encourage the sensitivity branch and specificity branch to generate consistent results for consensus labels and opposite results for disagreement labels, a consensus loss is proposed to constrain the output of the two branches. Meanwhile, the consistency/inconsistency between the prediction results of the two branches implies the image being an easy/hard case, which is further utilized to encourage the balanced fusion branch to concentrate more on the hard cases. Compared with models trained only with the final ground-truth labels, the proposed method using multi-rater consensus information has achieved superior performance, and it is also able to estimate the difficulty levels of individual input images when making the prediction.
Learning Crisp Edge Detector Using Logical Refinement Network
Jul 24, 2020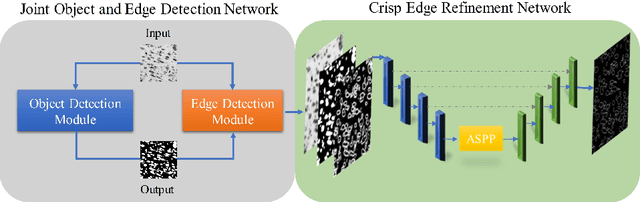
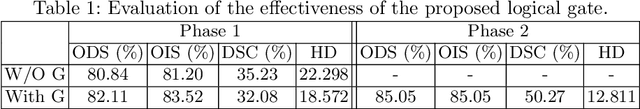
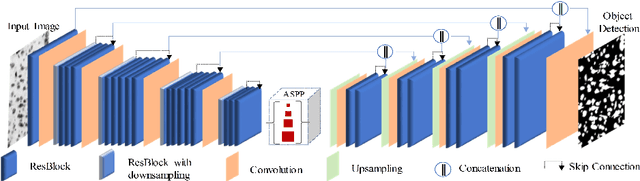
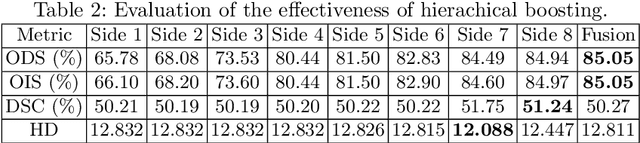
Abstract:Edge detection is a fundamental problem in different computer vision tasks. Recently, edge detection algorithms achieve satisfying improvement built upon deep learning. Although most of them report favorable evaluation scores, they often fail to accurately localize edges and give thick and blurry boundaries. In addition, most of them focus on 2D images and the challenging 3D edge detection is still under-explored. In this work, we propose a novel logical refinement network for crisp edge detection, which is motivated by the logical relationship between segmentation and edge maps and can be applied to both 2D and 3D images. The network consists of a joint object and edge detection network and a crisp edge refinement network, which predicts more accurate, clearer and thinner high quality binary edge maps without any post-processing. Extensive experiments are conducted on the 2D nuclei images from Kaggle 2018 Data Science Bowl and a private 3D microscopy images of a monkey brain, which show outstanding performance compared with state-of-the-art methods.
Leveraging Undiagnosed Data for Glaucoma Classification with Teacher-Student Learning
Jul 22, 2020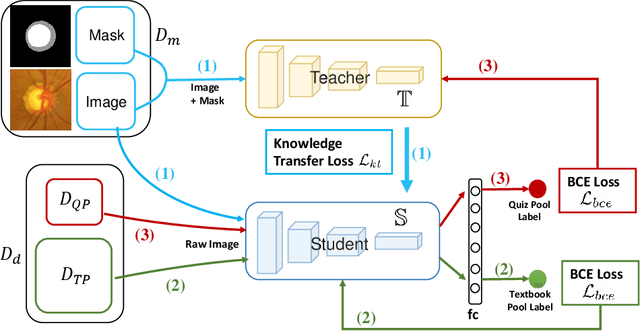
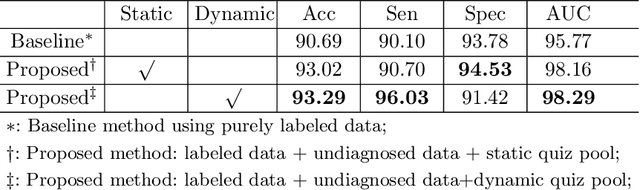
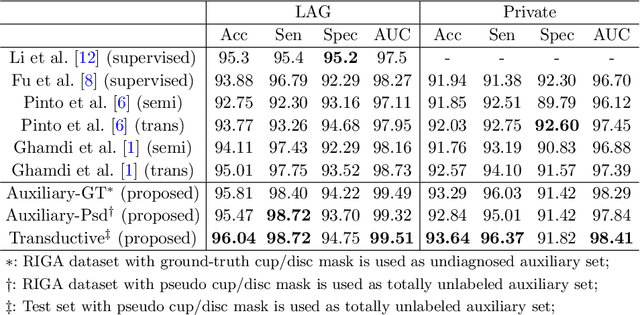
Abstract:Recently, deep learning has been adopted to the glaucoma classification task with performance comparable to that of human experts. However, a well trained deep learning model demands a large quantity of properly labeled data, which is relatively expensive since the accurate labeling of glaucoma requires years of specialist training. In order to alleviate this problem, we propose a glaucoma classification framework which takes advantage of not only the properly labeled images, but also undiagnosed images without glaucoma labels. To be more specific, the proposed framework is adapted from the teacher-student-learning paradigm. The teacher model encodes the wrapped information of undiagnosed images to a latent feature space, meanwhile the student model learns from the teacher through knowledge transfer to improve the glaucoma classification. For the model training procedure, we propose a novel training strategy that simulates the real-world teaching practice named as 'Learning To Teach with Knowledge Transfer (L2T-KT)', and establish a 'Quiz Pool' as the teacher's optimization target. Experiments show that the proposed framework is able to utilize the undiagnosed data effectively to improve the glaucoma prediction performance.
Instance-aware Self-supervised Learning for Nuclei Segmentation
Jul 22, 2020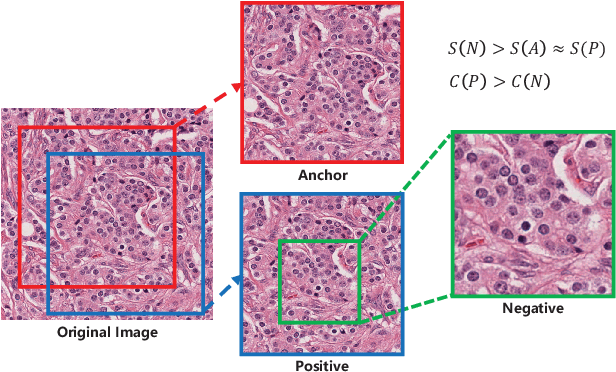
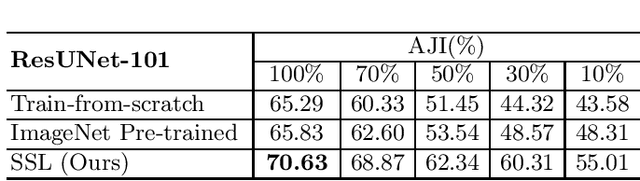
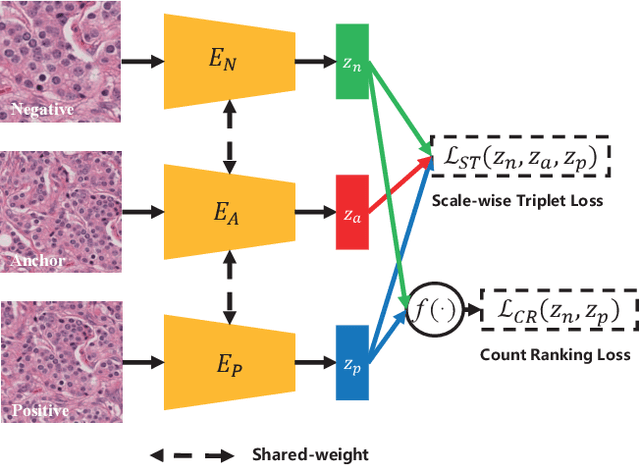
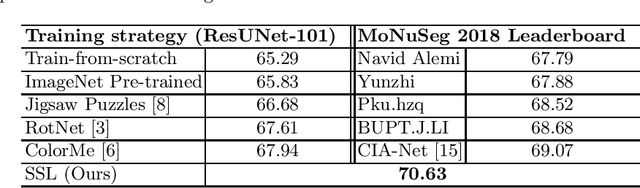
Abstract:Due to the wide existence and large morphological variances of nuclei, accurate nuclei instance segmentation is still one of the most challenging tasks in computational pathology. The annotating of nuclei instances, requiring experienced pathologists to manually draw the contours, is extremely laborious and expensive, which often results in the deficiency of annotated data. The deep learning based segmentation approaches, which highly rely on the quantity of training data, are difficult to fully demonstrate their capacity in this area. In this paper, we propose a novel self-supervised learning framework to deeply exploit the capacity of widely-used convolutional neural networks (CNNs) on the nuclei instance segmentation task. The proposed approach involves two sub-tasks (i.e., scale-wise triplet learning and count ranking), which enable neural networks to implicitly leverage the prior-knowledge of nuclei size and quantity, and accordingly mine the instance-aware feature representations from the raw data. Experimental results on the publicly available MoNuSeg dataset show that the proposed self-supervised learning approach can remarkably boost the segmentation accuracy of nuclei instance---a new state-of-the-art average Aggregated Jaccard Index (AJI) of 70.63%, is achieved by our self-supervised ResUNet-101. To our best knowledge, this is the first work focusing on the self-supervised learning for instance segmentation.
Comparing to Learn: Surpassing ImageNet Pretraining on Radiographs By Comparing Image Representations
Jul 22, 2020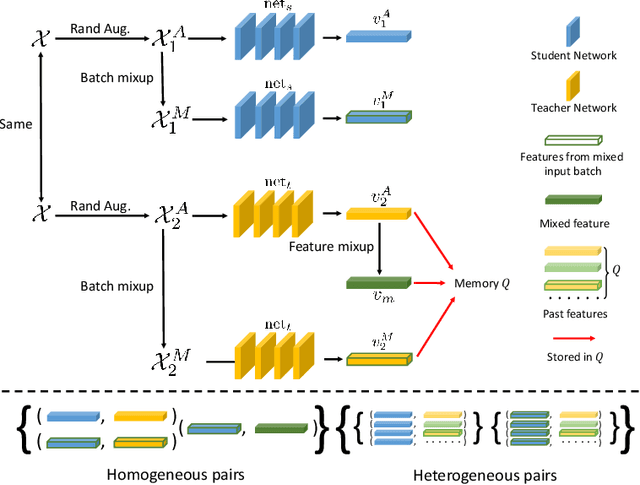
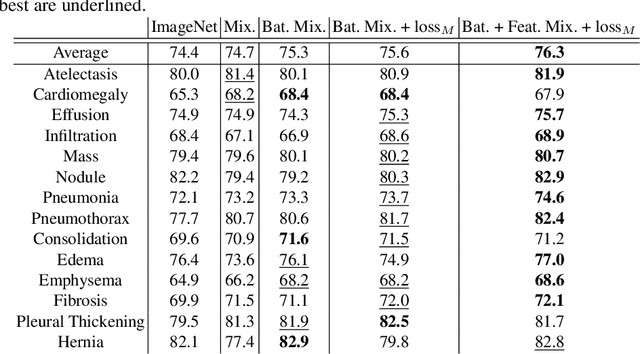

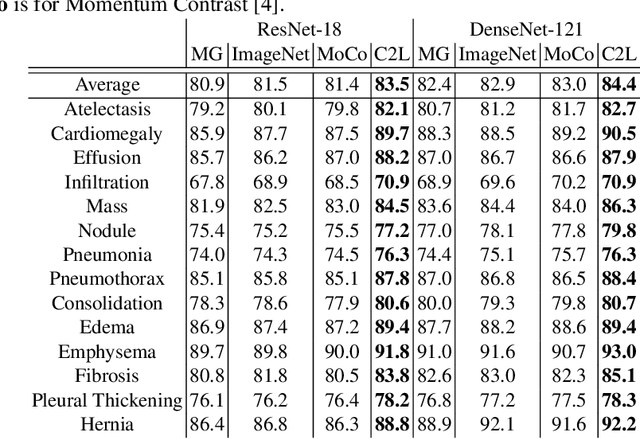
Abstract:In deep learning era, pretrained models play an important role in medical image analysis, in which ImageNet pretraining has been widely adopted as the best way. However, it is undeniable that there exists an obvious domain gap between natural images and medical images. To bridge this gap, we propose a new pretraining method which learns from 700k radiographs given no manual annotations. We call our method as Comparing to Learn (C2L) because it learns robust features by comparing different image representations. To verify the effectiveness of C2L, we conduct comprehensive ablation studies and evaluate it on different tasks and datasets. The experimental results on radiographs show that C2L can outperform ImageNet pretraining and previous state-of-the-art approaches significantly. Code and models are available.
GREEN: a Graph REsidual rE-ranking Network for Grading Diabetic Retinopathy
Jul 21, 2020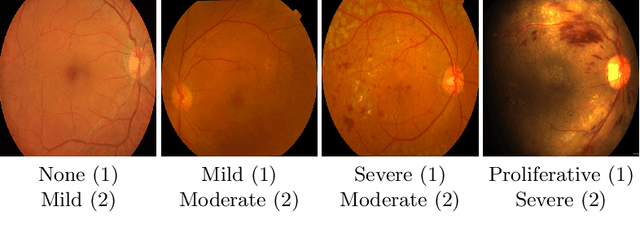
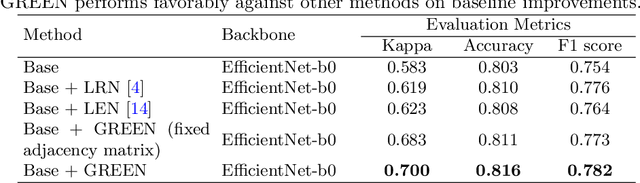
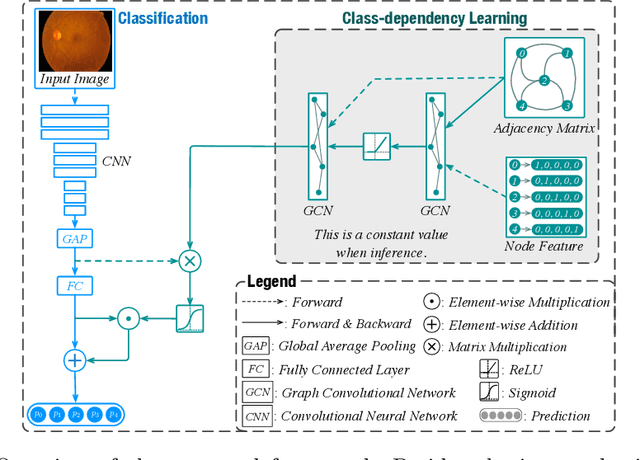
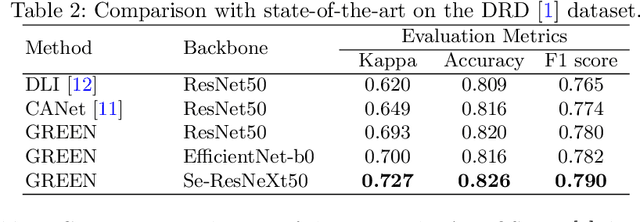
Abstract:The automatic grading of diabetic retinopathy (DR) facilitates medical diagnosis for both patients and physicians. Existing researches formulate DR grading as an image classification problem. As the stages/categories of DR correlate with each other, the relationship between different classes cannot be explicitly described via a one-hot label because it is empirically estimated by different physicians with different outcomes. This class correlation limits existing networks to achieve effective classification. In this paper, we propose a Graph REsidual rE-ranking Network (GREEN) to introduce a class dependency prior into the original image classification network. The class dependency prior is represented by a graph convolutional network with an adjacency matrix. This prior augments image classification pipeline by re-ranking classification results in a residual aggregation manner. Experiments on the standard benchmarks have shown that GREEN performs favorably against state-of-the-art approaches.
Distractor-Aware Neuron Intrinsic Learning for Generic 2D Medical Image Classifications
Jul 21, 2020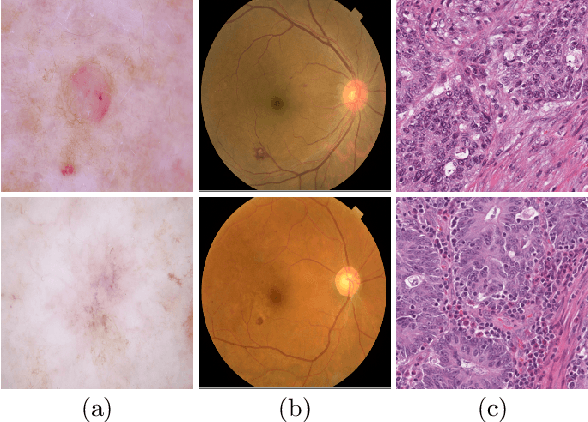
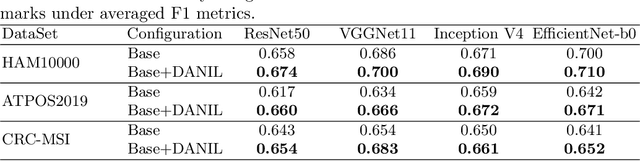
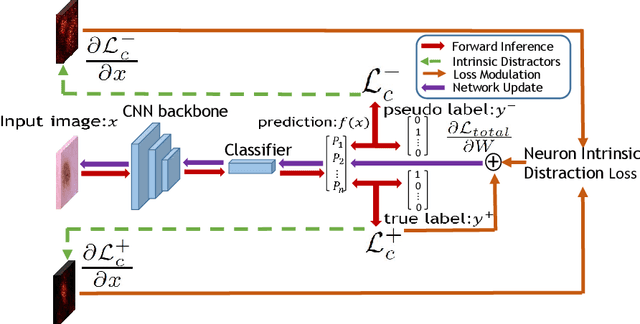
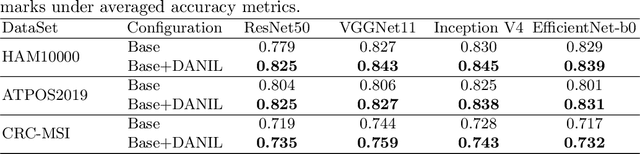
Abstract:Medical image analysis benefits Computer Aided Diagnosis (CADx). A fundamental analyzing approach is the classification of medical images, which serves for skin lesion diagnosis, diabetic retinopathy grading, and cancer classification on histological images. When learning these discriminative classifiers, we observe that the convolutional neural networks (CNNs) are vulnerable to distractor interference. This is due to the similar sample appearances from different categories (i.e., small inter-class distance). Existing attempts select distractors from input images by empirically estimating their potential effects to the classifier. The essences of how these distractors affect CNN classification are not known. In this paper, we explore distractors from the CNN feature space via proposing a neuron intrinsic learning method. We formulate a novel distractor-aware loss that encourages large distance between the original image and its distractor in the feature space. The novel loss is combined with the original classification loss to update network parameters by back-propagation. Neuron intrinsic learning first explores distractors crucial to the deep classifier and then uses them to robustify CNN inherently. Extensive experiments on medical image benchmark datasets indicate that the proposed method performs favorably against the state-of-the-art approaches.
A Macro-Micro Weakly-supervised Framework for AS-OCT Tissue Segmentation
Jul 20, 2020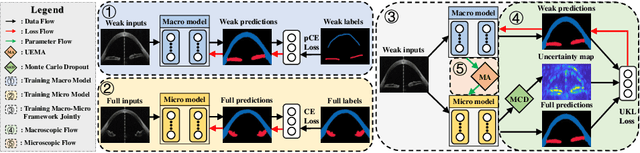
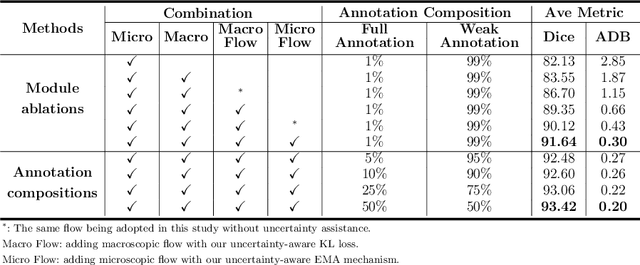
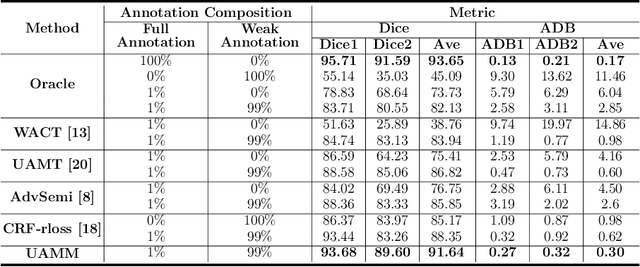
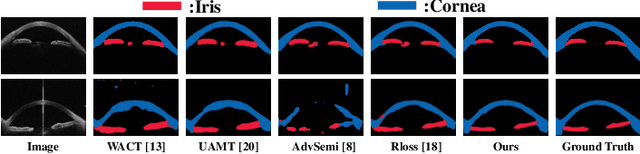
Abstract:Primary angle closure glaucoma (PACG) is the leading cause of irreversible blindness among Asian people. Early detection of PACG is essential, so as to provide timely treatment and minimize the vision loss. In the clinical practice, PACG is diagnosed by analyzing the angle between the cornea and iris with anterior segment optical coherence tomography (AS-OCT). The rapid development of deep learning technologies provides the feasibility of building a computer-aided system for the fast and accurate segmentation of cornea and iris tissues. However, the application of deep learning methods in the medical imaging field is still restricted by the lack of enough fully-annotated samples. In this paper, we propose a novel framework to segment the target tissues accurately for the AS-OCT images, by using the combination of weakly-annotated images (majority) and fully-annotated images (minority). The proposed framework consists of two models which provide reliable guidance for each other. In addition, uncertainty guided strategies are adopted to increase the accuracy and stability of the guidance. Detailed experiments on the publicly available AGE dataset demonstrate that the proposed framework outperforms the state-of-the-art semi-/weakly-supervised methods and has a comparable performance as the fully-supervised method. Therefore, the proposed method is demonstrated to be effective in exploiting information contained in the weakly-annotated images and has the capability to substantively relieve the annotation workload.
Deep Image Clustering with Category-Style Representation
Jul 20, 2020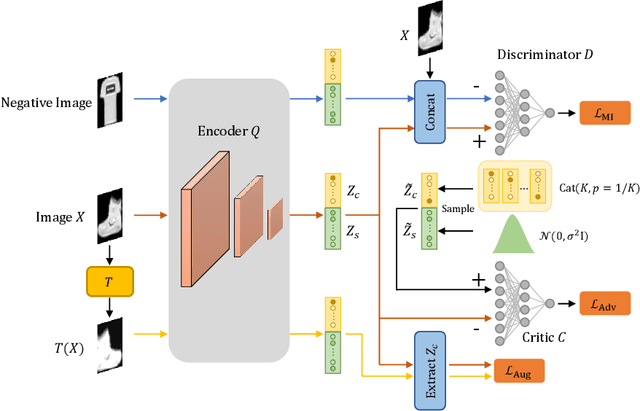
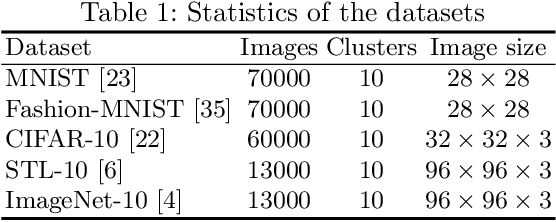
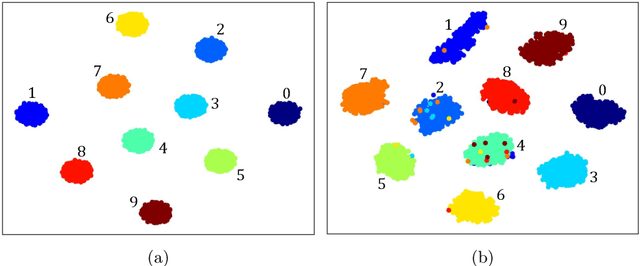
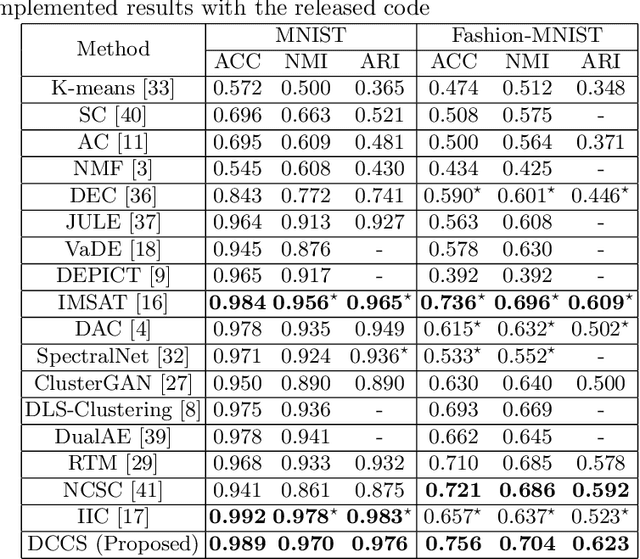
Abstract:Deep clustering which adopts deep neural networks to obtain optimal representations for clustering has been widely studied recently. In this paper, we propose a novel deep image clustering framework to learn a category-style latent representation in which the category information is disentangled from image style and can be directly used as the cluster assignment. To achieve this goal, mutual information maximization is applied to embed relevant information in the latent representation. Moreover, augmentation-invariant loss is employed to disentangle the representation into category part and style part. Last but not least, a prior distribution is imposed on the latent representation to ensure the elements of the category vector can be used as the probabilities over clusters. Comprehensive experiments demonstrate that the proposed approach outperforms state-of-the-art methods significantly on five public datasets.
Self-Loop Uncertainty: A Novel Pseudo-Label for Semi-Supervised Medical Image Segmentation
Jul 20, 2020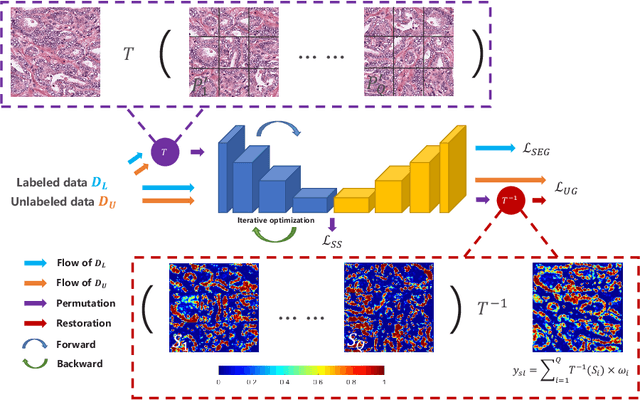
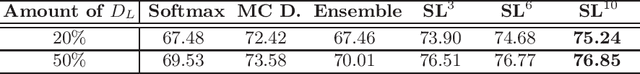
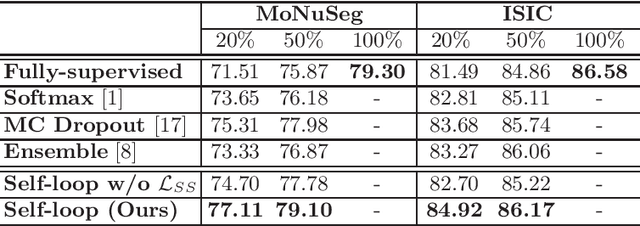
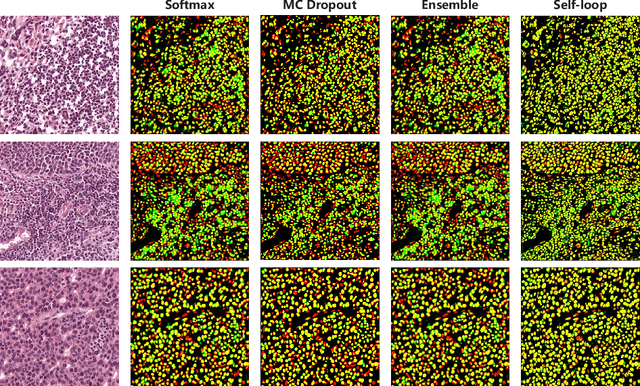
Abstract:Witnessing the success of deep learning neural networks in natural image processing, an increasing number of studies have been proposed to develop deep-learning-based frameworks for medical image segmentation. However, since the pixel-wise annotation of medical images is laborious and expensive, the amount of annotated data is usually deficient to well-train a neural network. In this paper, we propose a semi-supervised approach to train neural networks with limited labeled data and a large quantity of unlabeled images for medical image segmentation. A novel pseudo-label (namely self-loop uncertainty), generated by recurrently optimizing the neural network with a self-supervised task, is adopted as the ground-truth for the unlabeled images to augment the training set and boost the segmentation accuracy. The proposed self-loop uncertainty can be seen as an approximation of the uncertainty estimation yielded by ensembling multiple models with a significant reduction of inference time. Experimental results on two publicly available datasets demonstrate the effectiveness of our semi-supervied approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge