João L. Vilaça
Point Tracking in Surgery--The 2024 Surgical Tattoos in Infrared (STIR) Challenge
Mar 31, 2025Abstract:Understanding tissue motion in surgery is crucial to enable applications in downstream tasks such as segmentation, 3D reconstruction, virtual tissue landmarking, autonomous probe-based scanning, and subtask autonomy. Labeled data are essential to enabling algorithms in these downstream tasks since they allow us to quantify and train algorithms. This paper introduces a point tracking challenge to address this, wherein participants can submit their algorithms for quantification. The submitted algorithms are evaluated using a dataset named surgical tattoos in infrared (STIR), with the challenge aptly named the STIR Challenge 2024. The STIR Challenge 2024 comprises two quantitative components: accuracy and efficiency. The accuracy component tests the accuracy of algorithms on in vivo and ex vivo sequences. The efficiency component tests the latency of algorithm inference. The challenge was conducted as a part of MICCAI EndoVis 2024. In this challenge, we had 8 total teams, with 4 teams submitting before and 4 submitting after challenge day. This paper details the STIR Challenge 2024, which serves to move the field towards more accurate and efficient algorithms for spatial understanding in surgery. In this paper we summarize the design, submissions, and results from the challenge. The challenge dataset is available here: https://zenodo.org/records/14803158 , and the code for baseline models and metric calculation is available here: https://github.com/athaddius/STIRMetrics
Exploring Optical Flow Inclusion into nnU-Net Framework for Surgical Instrument Segmentation
Mar 15, 2024Abstract:Surgical instrument segmentation in laparoscopy is essential for computer-assisted surgical systems. Despite the Deep Learning progress in recent years, the dynamic setting of laparoscopic surgery still presents challenges for precise segmentation. The nnU-Net framework excelled in semantic segmentation analyzing single frames without temporal information. The framework's ease of use, including its ability to be automatically configured, and its low expertise requirements, have made it a popular base framework for comparisons. Optical flow (OF) is a tool commonly used in video tasks to estimate motion and represent it in a single frame, containing temporal information. This work seeks to employ OF maps as an additional input to the nnU-Net architecture to improve its performance in the surgical instrument segmentation task, taking advantage of the fact that instruments are the main moving objects in the surgical field. With this new input, the temporal component would be indirectly added without modifying the architecture. Using CholecSeg8k dataset, three different representations of movement were estimated and used as new inputs, comparing them with a baseline model. Results showed that the use of OF maps improves the detection of classes with high movement, even when these are scarce in the dataset. To further improve performance, future work may focus on implementing other OF-preserving augmentations.
Why is the winner the best?
Mar 30, 2023



Abstract:International benchmarking competitions have become fundamental for the comparative performance assessment of image analysis methods. However, little attention has been given to investigating what can be learnt from these competitions. Do they really generate scientific progress? What are common and successful participation strategies? What makes a solution superior to a competing method? To address this gap in the literature, we performed a multi-center study with all 80 competitions that were conducted in the scope of IEEE ISBI 2021 and MICCAI 2021. Statistical analyses performed based on comprehensive descriptions of the submitted algorithms linked to their rank as well as the underlying participation strategies revealed common characteristics of winning solutions. These typically include the use of multi-task learning (63%) and/or multi-stage pipelines (61%), and a focus on augmentation (100%), image preprocessing (97%), data curation (79%), and postprocessing (66%). The "typical" lead of a winning team is a computer scientist with a doctoral degree, five years of experience in biomedical image analysis, and four years of experience in deep learning. Two core general development strategies stood out for highly-ranked teams: the reflection of the metrics in the method design and the focus on analyzing and handling failure cases. According to the organizers, 43% of the winning algorithms exceeded the state of the art but only 11% completely solved the respective domain problem. The insights of our study could help researchers (1) improve algorithm development strategies when approaching new problems, and (2) focus on open research questions revealed by this work.
CholecTriplet2022: Show me a tool and tell me the triplet -- an endoscopic vision challenge for surgical action triplet detection
Feb 13, 2023



Abstract:Formalizing surgical activities as triplets of the used instruments, actions performed, and target anatomies is becoming a gold standard approach for surgical activity modeling. The benefit is that this formalization helps to obtain a more detailed understanding of tool-tissue interaction which can be used to develop better Artificial Intelligence assistance for image-guided surgery. Earlier efforts and the CholecTriplet challenge introduced in 2021 have put together techniques aimed at recognizing these triplets from surgical footage. Estimating also the spatial locations of the triplets would offer a more precise intraoperative context-aware decision support for computer-assisted intervention. This paper presents the CholecTriplet2022 challenge, which extends surgical action triplet modeling from recognition to detection. It includes weakly-supervised bounding box localization of every visible surgical instrument (or tool), as the key actors, and the modeling of each tool-activity in the form of <instrument, verb, target> triplet. The paper describes a baseline method and 10 new deep learning algorithms presented at the challenge to solve the task. It also provides thorough methodological comparisons of the methods, an in-depth analysis of the obtained results, their significance, and useful insights for future research directions and applications in surgery.
Fetal Brain Tissue Annotation and Segmentation Challenge Results
Apr 20, 2022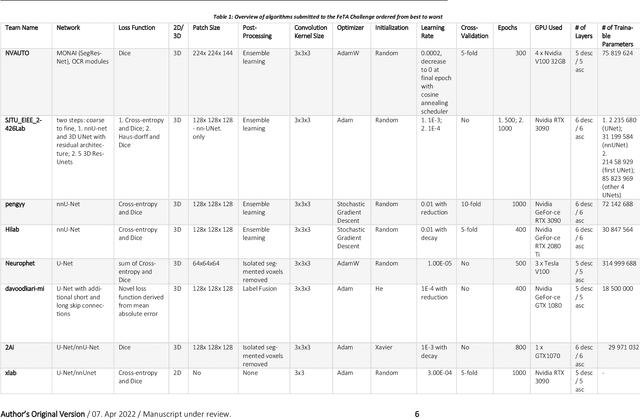
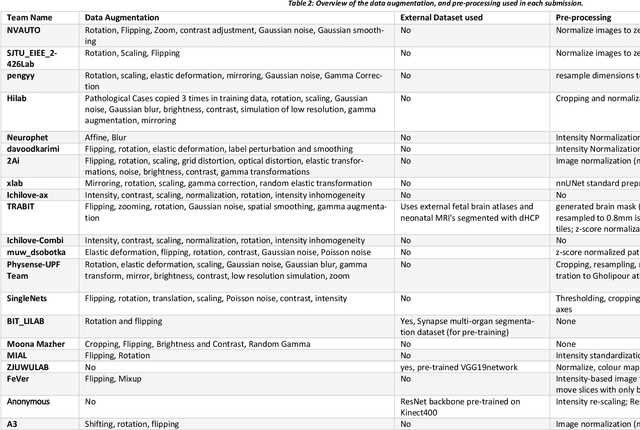
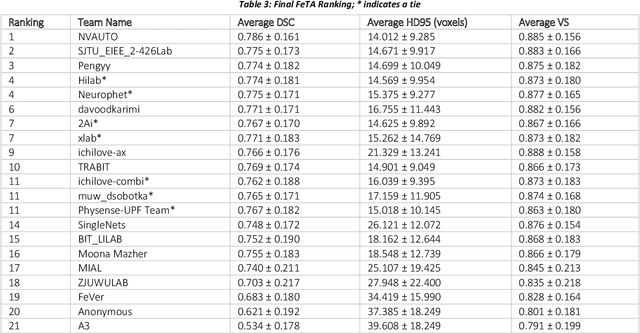
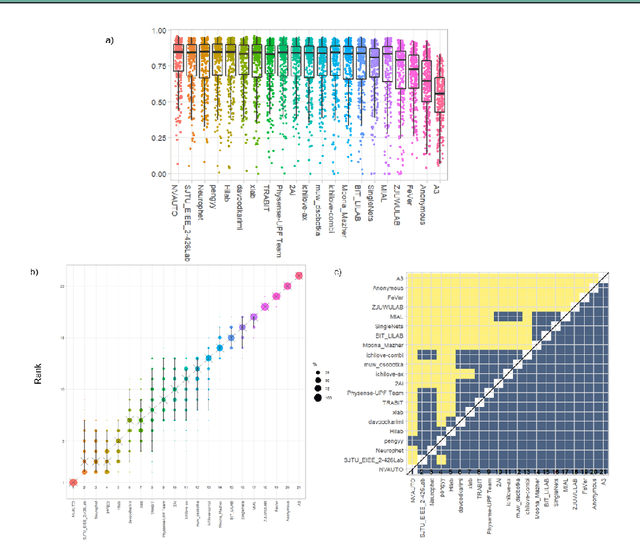
Abstract:In-utero fetal MRI is emerging as an important tool in the diagnosis and analysis of the developing human brain. Automatic segmentation of the developing fetal brain is a vital step in the quantitative analysis of prenatal neurodevelopment both in the research and clinical context. However, manual segmentation of cerebral structures is time-consuming and prone to error and inter-observer variability. Therefore, we organized the Fetal Tissue Annotation (FeTA) Challenge in 2021 in order to encourage the development of automatic segmentation algorithms on an international level. The challenge utilized FeTA Dataset, an open dataset of fetal brain MRI reconstructions segmented into seven different tissues (external cerebrospinal fluid, grey matter, white matter, ventricles, cerebellum, brainstem, deep grey matter). 20 international teams participated in this challenge, submitting a total of 21 algorithms for evaluation. In this paper, we provide a detailed analysis of the results from both a technical and clinical perspective. All participants relied on deep learning methods, mainly U-Nets, with some variability present in the network architecture, optimization, and image pre- and post-processing. The majority of teams used existing medical imaging deep learning frameworks. The main differences between the submissions were the fine tuning done during training, and the specific pre- and post-processing steps performed. The challenge results showed that almost all submissions performed similarly. Four of the top five teams used ensemble learning methods. However, one team's algorithm performed significantly superior to the other submissions, and consisted of an asymmetrical U-Net network architecture. This paper provides a first of its kind benchmark for future automatic multi-tissue segmentation algorithms for the developing human brain in utero.
CholecTriplet2021: A benchmark challenge for surgical action triplet recognition
Apr 10, 2022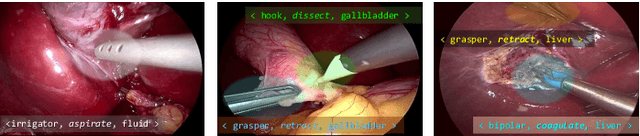
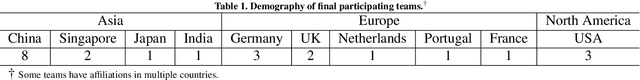
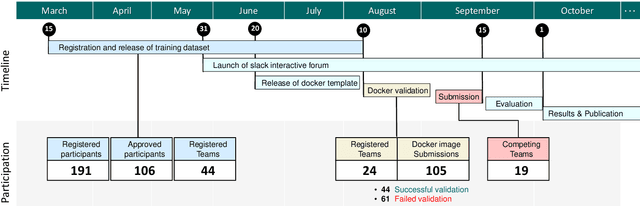
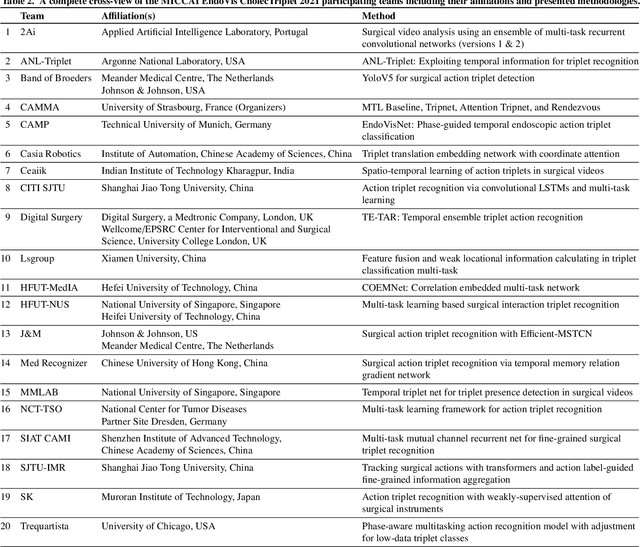
Abstract:Context-aware decision support in the operating room can foster surgical safety and efficiency by leveraging real-time feedback from surgical workflow analysis. Most existing works recognize surgical activities at a coarse-grained level, such as phases, steps or events, leaving out fine-grained interaction details about the surgical activity; yet those are needed for more helpful AI assistance in the operating room. Recognizing surgical actions as triplets of <instrument, verb, target> combination delivers comprehensive details about the activities taking place in surgical videos. This paper presents CholecTriplet2021: an endoscopic vision challenge organized at MICCAI 2021 for the recognition of surgical action triplets in laparoscopic videos. The challenge granted private access to the large-scale CholecT50 dataset, which is annotated with action triplet information. In this paper, we present the challenge setup and assessment of the state-of-the-art deep learning methods proposed by the participants during the challenge. A total of 4 baseline methods from the challenge organizers and 19 new deep learning algorithms by competing teams are presented to recognize surgical action triplets directly from surgical videos, achieving mean average precision (mAP) ranging from 4.2% to 38.1%. This study also analyzes the significance of the results obtained by the presented approaches, performs a thorough methodological comparison between them, in-depth result analysis, and proposes a novel ensemble method for enhanced recognition. Our analysis shows that surgical workflow analysis is not yet solved, and also highlights interesting directions for future research on fine-grained surgical activity recognition which is of utmost importance for the development of AI in surgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge