Shanshan Cui
A Benchmark for Studying Diabetic Retinopathy: Segmentation, Grading, and Transferability
Aug 30, 2020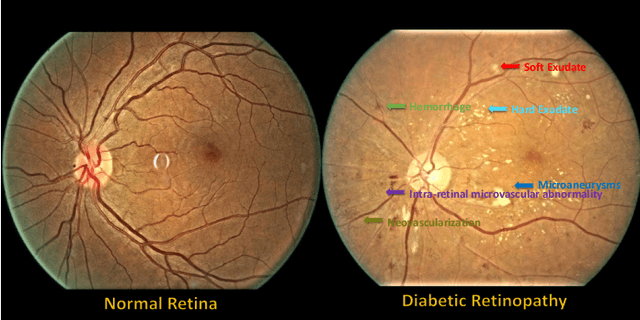
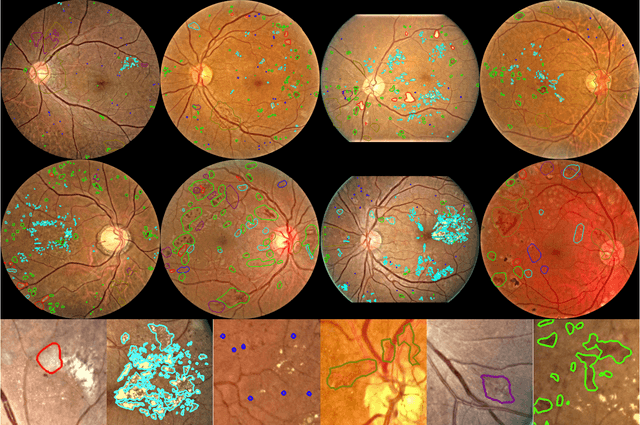
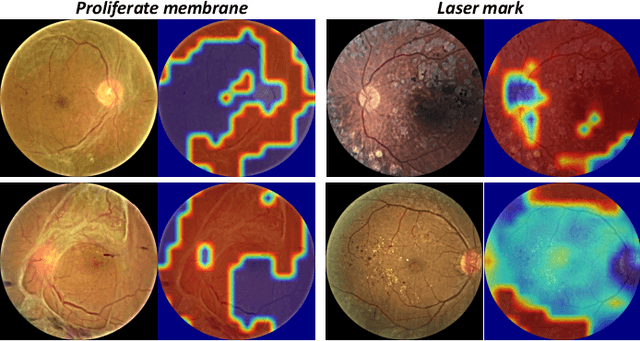
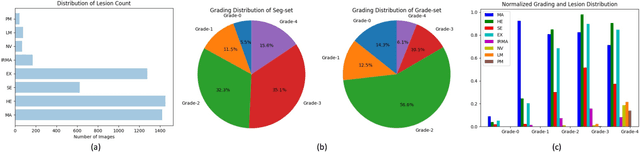
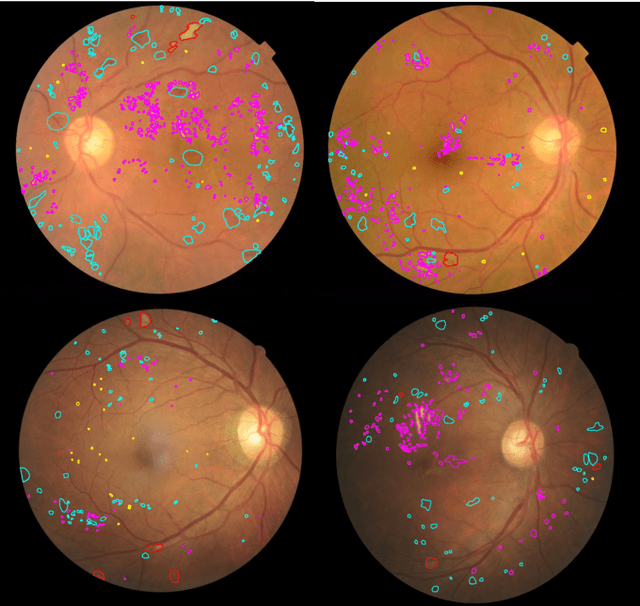
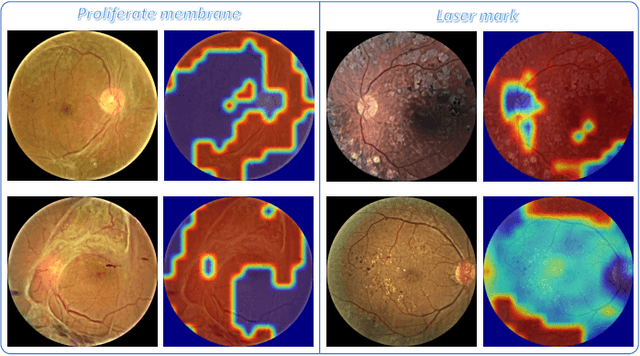
Abstract:People with diabetes are at risk of developing an eye disease called diabetic retinopathy (DR). This disease occurs when high blood glucose levels cause damage to blood vessels in the retina. Computer-aided DR diagnosis is a promising tool for early detection of DR and severity grading, due to the great success of deep learning. However, most current DR diagnosis systems do not achieve satisfactory performance or interpretability for ophthalmologists, due to the lack of training data with consistent and fine-grained annotations. To address this problem, we construct a large fine-grained annotated DR dataset containing 2,842 images (FGADR). This dataset has 1,842 images with pixel-level DR-related lesion annotations, and 1,000 images with image-level labels graded by six board-certified ophthalmologists with intra-rater consistency. The proposed dataset will enable extensive studies on DR diagnosis. We set up three benchmark tasks for evaluation: 1. DR lesion segmentation; 2. DR grading by joint classification and segmentation; 3. Transfer learning for ocular multi-disease identification. Moreover, a novel inductive transfer learning method is introduced for the third task. Extensive experiments using different state-of-the-art methods are conducted on our FGADR dataset, which can serve as baselines for future research.
DR-GAN: Conditional Generative Adversarial Network for Fine-Grained Lesion Synthesis on Diabetic Retinopathy Images
Dec 10, 2019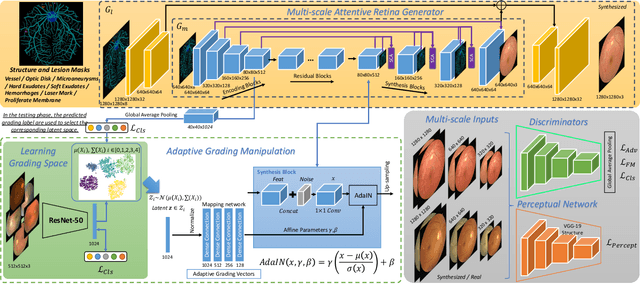
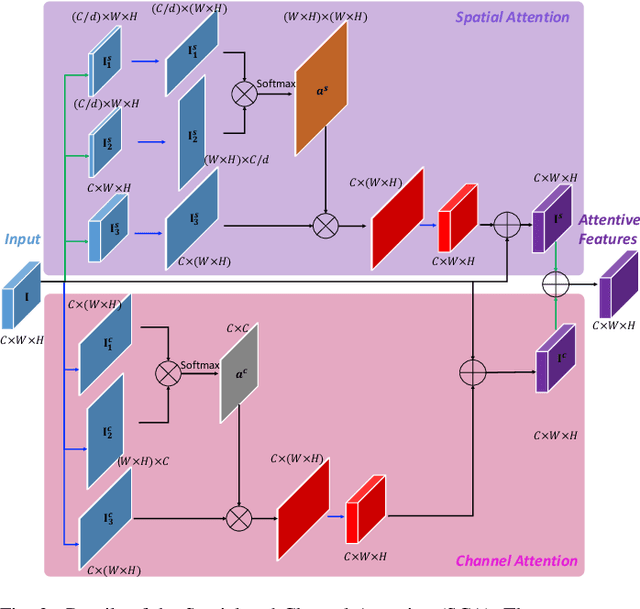
Abstract:Diabetic retinopathy (DR) is a complication of diabetes that severely affects eyes. It can be graded into five levels of severity according to international protocol. However, optimizing a grading model to have strong generalizability requires a large amount of balanced training data, which is difficult to collect particularly for the high severity levels. Typical data augmentation methods, including random flipping and rotation, cannot generate data with high diversity. In this paper, we propose a diabetic retinopathy generative adversarial network (DR-GAN) to synthesize high-resolution fundus images which can be manipulated with arbitrary grading and lesion information. Thus, large-scale generated data can be used for more meaningful augmentation to train a DR grading and lesion segmentation model. The proposed retina generator is conditioned on the structural and lesion masks, as well as adaptive grading vectors sampled from the latent grading space, which can be adopted to control the synthesized grading severity. Moreover, a multi-scale spatial and channel attention module is devised to improve the generation ability to synthesize details. Multi-scale discriminators are designed to operate from large to small receptive fields, and joint adversarial losses are adopted to optimize the whole network in an end-to-end manner. With extensive experiments evaluated on the EyePACS dataset connected to Kaggle, as well as our private dataset (SKA - will be released once get official permission), we validate the effectiveness of our method, which can both synthesize highly realistic (1280 x 1280) controllable fundus images and contribute to the DR grading task.
Evaluation of Retinal Image Quality Assessment Networks in Different Color-spaces
Jul 18, 2019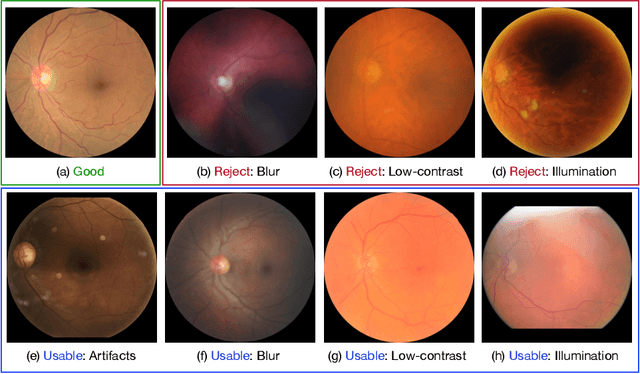
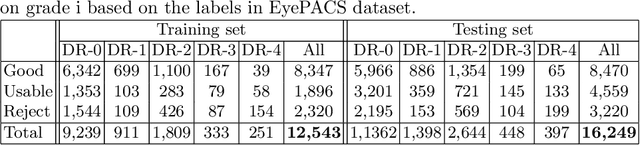
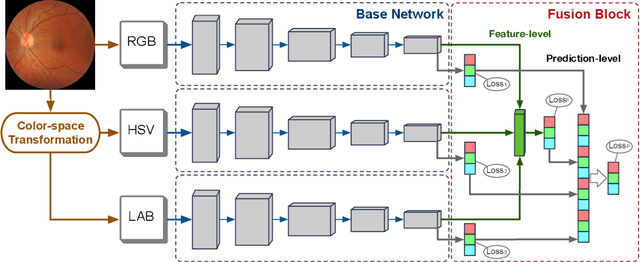
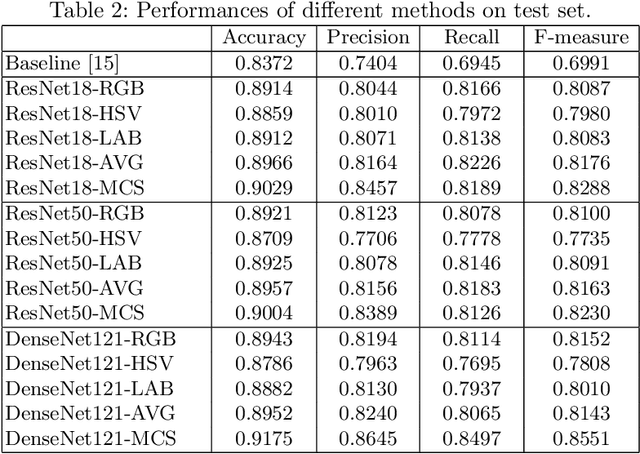
Abstract:Retinal image quality assessment (RIQA) is essential for controlling the quality of retinal imaging and guaranteeing the reliability of diagnoses by ophthalmologists or automated analysis systems. Existing RIQA methods focus on the RGB color-space and are developed based on small datasets with binary quality labels (i.e., `Accept' and `Reject'). In this paper, we first re-annotate an Eye-Quality (EyeQ) dataset with 28,792 retinal images from the EyePACS dataset, based on a three-level quality grading system (i.e., `Good', `Usable' and `Reject') for evaluating RIQA methods. Our RIQA dataset is characterized by its large-scale size, multi-level grading, and multi-modality. Then, we analyze the influences on RIQA of different color-spaces, and propose a simple yet efficient deep network, named Multiple Color-space Fusion Network (MCF-Net), which integrates the different color-space representations at both a feature-level and prediction-level to predict image quality grades. Experiments on our EyeQ dataset show that our MCF-Net obtains a state-of-the-art performance, outperforming the other deep learning methods. Furthermore, we also evaluate diabetic retinopathy (DR) detection methods on images of different quality, and demonstrate that the performances of automated diagnostic systems are highly dependent on image quality.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge