Qunxi Dong
LLM-as-a-Supervisor: Mistaken Therapeutic Behaviors Trigger Targeted Supervisory Feedback
Aug 12, 2025



Abstract:Although large language models (LLMs) hold significant promise in psychotherapy, their direct application in patient-facing scenarios raises ethical and safety concerns. Therefore, this work shifts towards developing an LLM as a supervisor to train real therapists. In addition to the privacy of clinical therapist training data, a fundamental contradiction complicates the training of therapeutic behaviors: clear feedback standards are necessary to ensure a controlled training system, yet there is no absolute "gold standard" for appropriate therapeutic behaviors in practice. In contrast, many common therapeutic mistakes are universal and identifiable, making them effective triggers for targeted feedback that can serve as clearer evidence. Motivated by this, we create a novel therapist-training paradigm: (1) guidelines for mistaken behaviors and targeted correction strategies are first established as standards; (2) a human-in-the-loop dialogue-feedback dataset is then constructed, where a mistake-prone agent intentionally makes standard mistakes during interviews naturally, and a supervisor agent locates and identifies mistakes and provides targeted feedback; (3) after fine-tuning on this dataset, the final supervisor model is provided for real therapist training. The detailed experimental results of automated, human and downstream assessments demonstrate that models fine-tuned on our dataset MATE, can provide high-quality feedback according to the clinical guideline, showing significant potential for the therapist training scenario.
DECIDER: A Rule-Controllable Decoding Strategy for Language Generation by Imitating Dual-System Cognitive Theory
Mar 04, 2024Abstract:Lexicon-based constrained decoding approaches aim to control the meaning or style of the generated text through certain target concepts. Existing approaches over-focus the targets themselves, leading to a lack of high-level reasoning about how to achieve them. However, human usually tackles tasks by following certain rules that not only focuses on the targets but also on semantically relevant concepts that induce the occurrence of targets. In this work, we present DECIDER, a rule-controllable decoding strategy for constrained language generation inspired by dual-system cognitive theory. Specifically, in DECIDER, a pre-trained language model (PLM) is equiped with a logic reasoner that takes high-level rules as input. Then, the DECIDER allows rule signals to flow into the PLM at each decoding step. Extensive experimental results demonstrate that DECIDER can effectively follow given rules to guide generation direction toward the targets in a more human-like manner.
Developing Univariate Neurodegeneration Biomarkers with Low-Rank and Sparse Subspace Decomposition
Oct 26, 2020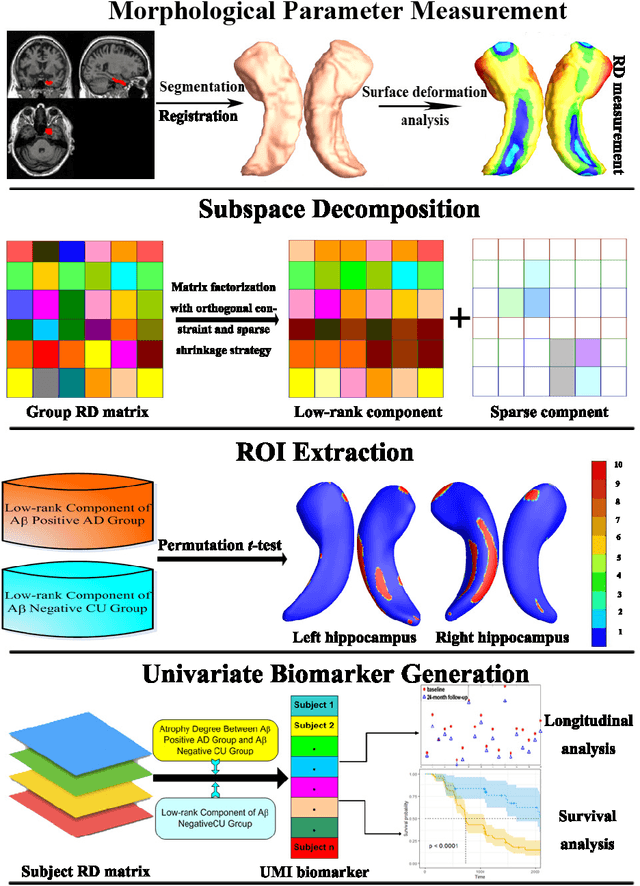
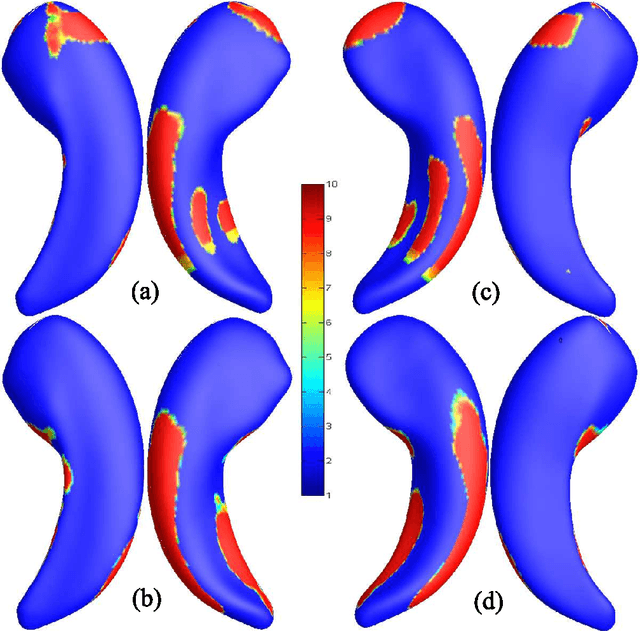
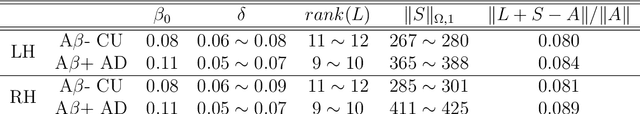
Abstract:Cognitive decline due to Alzheimer's disease (AD) is closely associated with brain structure alterations captured by structural magnetic resonance imaging (sMRI). It supports the validity to develop sMRI-based univariate neurodegeneration biomarkers (UNB). However, existing UNB work either fails to model large group variances or does not capture AD dementia (ADD) induced changes. We propose a novel low-rank and sparse subspace decomposition method capable of stably quantifying the morphological changes induced by ADD. Specifically, we propose a numerically efficient rank minimization mechanism to extract group common structure and impose regularization constraints to encode the original 3D morphometry connectivity. Further, we generate regions-of-interest (ROI) with group difference study between common subspaces of $A\beta+$ AD and $A\beta-$ cognitively unimpaired (CU) groups. A univariate morphometry index (UMI) is constructed from these ROIs by summarizing individual morphological characteristics weighted by normalized difference between $A\beta+$ AD and $A\beta-$ CU groups. We use hippocampal surface radial distance feature to compute the UMIs and validate our work in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort. With hippocampal UMIs, the estimated minimum sample sizes needed to detect a 25$\%$ reduction in the mean annual change with 80$\%$ power and two-tailed $P=0.05$ are 116, 279 and 387 for the longitudinal $A\beta+$ AD, $A\beta+$ mild cognitive impairment (MCI) and $A\beta+$ CU groups, respectively. Additionally, for MCI patients, UMIs well correlate with hazard ratio of conversion to AD ($4.3$, $95\%$ CI=$2.3-8.2$) within 18 months. Our experimental results outperform traditional hippocampal volume measures and suggest the application of UMI as a potential UNB.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge