Hoifung Poon
Context-faithful Prompting for Large Language Models
Mar 20, 2023Abstract:Large language models (LLMs) encode parametric knowledge about world facts and have shown remarkable performance in knowledge-driven NLP tasks. However, their reliance on parametric knowledge may cause them to overlook contextual cues, leading to incorrect predictions in context-sensitive NLP tasks (e.g., knowledge acquisition tasks). In this paper, we seek to assess and enhance LLMs' contextual faithfulness in two aspects: knowledge conflict and prediction with abstention. We demonstrate that LLMs' faithfulness can be significantly improved using carefully designed prompting strategies. In particular, we identify opinion-based prompts and counterfactual demonstrations as the most effective methods. Opinion-based prompts reframe the context as a narrator's statement and inquire about the narrator's opinions, while counterfactual demonstrations use instances containing false facts to improve faithfulness in knowledge conflict situations. Neither technique requires additional training. We conduct experiments on three datasets of two standard NLP tasks, machine reading comprehension and relation extraction, and the results demonstrate significant improvement in faithfulness to contexts.
Large-Scale Domain-Specific Pretraining for Biomedical Vision-Language Processing
Mar 02, 2023Abstract:Contrastive pretraining on parallel image-text data has attained great success in vision-language processing (VLP), as exemplified by CLIP and related methods. However, prior explorations tend to focus on general domains in the web. Biomedical images and text are rather different, but publicly available datasets are small and skew toward chest X-ray, thus severely limiting progress. In this paper, we conducted by far the largest study on biomedical VLP, using 15 million figure-caption pairs extracted from biomedical research articles in PubMed Central. Our dataset (PMC-15M) is two orders of magnitude larger than existing biomedical image-text datasets such as MIMIC-CXR, and spans a diverse range of biomedical images. The standard CLIP method is suboptimal for the biomedical domain. We propose BiomedCLIP with domain-specific adaptations tailored to biomedical VLP. We conducted extensive experiments and ablation studies on standard biomedical imaging tasks from retrieval to classification to visual question-answering (VQA). BiomedCLIP established new state of the art in a wide range of standard datasets, substantially outperformed prior VLP approaches. Surprisingly, BiomedCLIP even outperformed radiology-specific state-of-the-art models such as BioViL on radiology-specific tasks such as RSNA pneumonia detection, thus highlighting the utility in large-scale pretraining across all biomedical image types. We will release our models at https://aka.ms/biomedclip to facilitate future research in biomedical VLP.
BLIAM: Literature-based Data Synthesis for Synergistic Drug Combination Prediction
Feb 16, 2023Abstract:Language models pre-trained on scientific literature corpora have substantially advanced scientific discovery by offering high-quality feature representations for downstream applications. However, these features are often not interpretable, and thus can reveal limited insights to domain experts. Instead of obtaining features from language models, we propose BLIAM, a literature-based data synthesis approach to directly generate training data points that are interpretable and model-agnostic to downstream applications. The key idea of BLIAM is to create prompts using existing training data and then use these prompts to synthesize new data points. BLIAM performs these two steps iteratively as new data points will define more informative prompts and new prompts will in turn synthesize more accurate data points. Notably, literature-based data augmentation might introduce data leakage since labels of test data points in downstream applications might have already been mentioned in the language model corpus. To prevent such leakage, we introduce GDSC-combo, a large-scale drug combination discovery dataset that was published after the biomedical language model was trained. We found that BLIAM substantially outperforms a non-augmented approach and manual prompting in this rigorous data split setting. BLIAM can be further used to synthesize data points for novel drugs and cell lines that were not even measured in biomedical experiments. In addition to the promising prediction performance, the data points synthesized by BLIAM are interpretable and model-agnostic, enabling in silico augmentation for in vitro experiments.
Continual Contrastive Finetuning Improves Low-Resource Relation Extraction
Dec 21, 2022Abstract:Relation extraction (RE), which has relied on structurally annotated corpora for model training, has been particularly challenging in low-resource scenarios and domains. Recent literature has tackled low-resource RE by self-supervised learning, where the solution involves pretraining the relation embedding by RE-based objective and finetuning on labeled data by classification-based objective. However, a critical challenge to this approach is the gap in objectives, which prevents the RE model from fully utilizing the knowledge in pretrained representations. In this paper, we aim at bridging the gap and propose to pretrain and finetune the RE model using consistent objectives of contrastive learning. Since in this kind of representation learning paradigm, one relation may easily form multiple clusters in the representation space, we further propose a multi-center contrastive loss that allows one relation to form multiple clusters to better align with pretraining. Experiments on two document-level RE datasets, BioRED and Re-DocRED, demonstrate the effectiveness of our method. Particularly, when using 1% end-task training data, our method outperforms PLM-based RE classifier by 10.5% and 5.8% on the two datasets, respectively.
BioGPT: Generative Pre-trained Transformer for Biomedical Text Generation and Mining
Oct 19, 2022
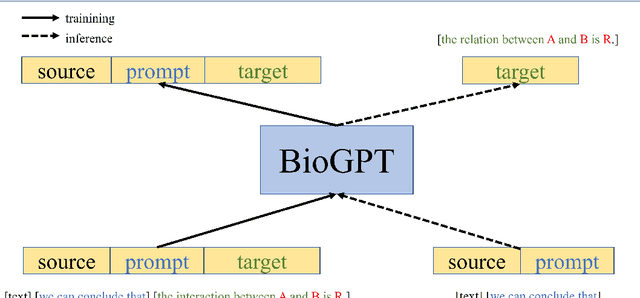
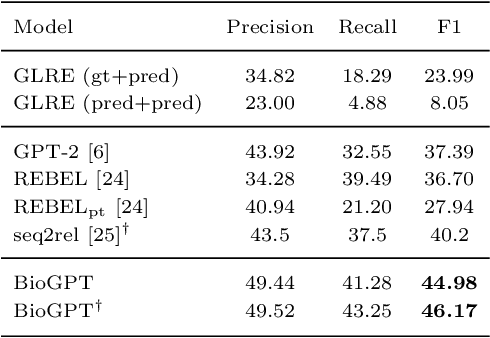
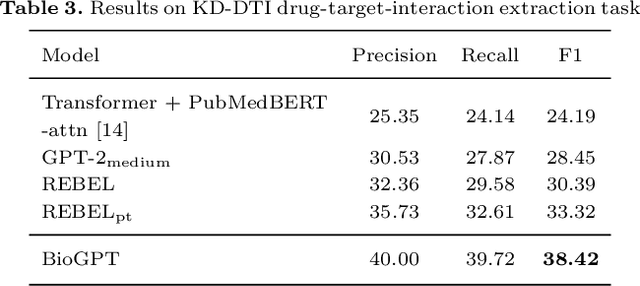
Abstract:Pre-trained language models have attracted increasing attention in the biomedical domain, inspired by their great success in the general natural language domain. Among the two main branches of pre-trained language models in the general language domain, i.e., BERT (and its variants) and GPT (and its variants), the first one has been extensively studied in the biomedical domain, such as BioBERT and PubMedBERT. While they have achieved great success on a variety of discriminative downstream biomedical tasks, the lack of generation ability constrains their application scope. In this paper, we propose BioGPT, a domain-specific generative Transformer language model pre-trained on large scale biomedical literature. We evaluate BioGPT on six biomedical NLP tasks and demonstrate that our model outperforms previous models on most tasks. Especially, we get 44.98%, 38.42% and 40.76% F1 score on BC5CDR, KD-DTI and DDI end-to-end relation extraction tasks respectively, and 78.2% accuracy on PubMedQA, creating a new record. Our case study on text generation further demonstrates the advantage of BioGPT on biomedical literature to generate fluent descriptions for biomedical terms. Code is available at https://github.com/microsoft/BioGPT.
* Published at Briefings in Bioinformatics. Code is available at https://github.com/microsoft/BioGPT
Optimizing Bi-Encoder for Named Entity Recognition via Contrastive Learning
Aug 30, 2022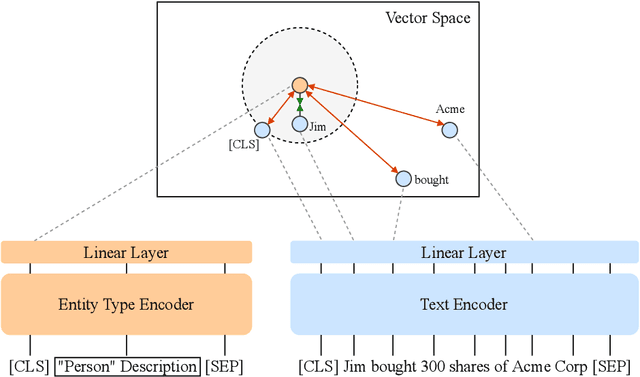
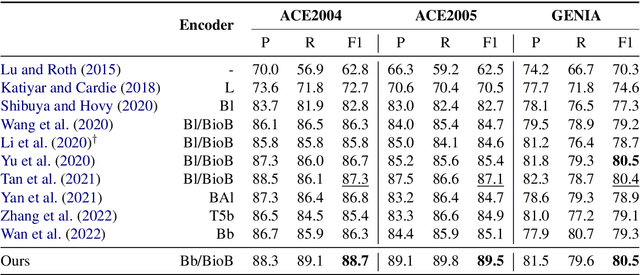
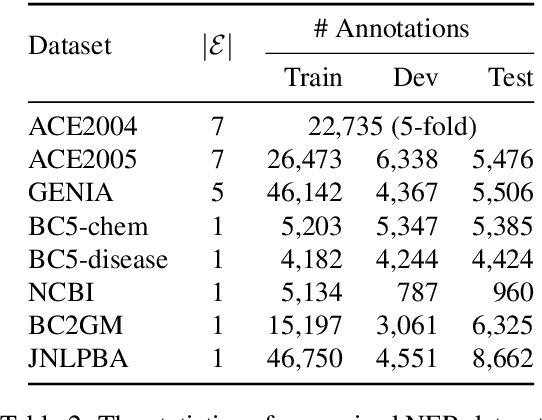
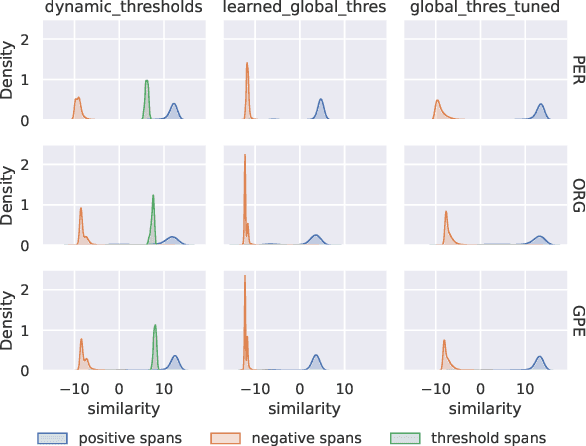
Abstract:We present an efficient bi-encoder framework for named entity recognition (NER), which applies contrastive learning to map candidate text spans and entity types into the same vector representation space. Prior work predominantly approaches NER as sequence labeling or span classification. We instead frame NER as a metric learning problem that maximizes the similarity between the vector representations of an entity mention and its type. This makes it easy to handle nested and flat NER alike, and can better leverage noisy self-supervision signals. A major challenge to this bi-encoder formulation for NER lies in separating non-entity spans from entity mentions. Instead of explicitly labeling all non-entity spans as the same class Outside (O) as in most prior methods, we introduce a novel dynamic thresholding loss, which is learned in conjunction with the standard contrastive loss. Experiments show that our method performs well in both supervised and distantly supervised settings, for nested and flat NER alike, establishing new state of the art across standard datasets in the general domain (e.g., ACE2004, ACE2005) and high-value verticals such as biomedicine (e.g., GENIA, NCBI, BC5CDR, JNLPBA).
Making the Most of Text Semantics to Improve Biomedical Vision--Language Processing
Apr 21, 2022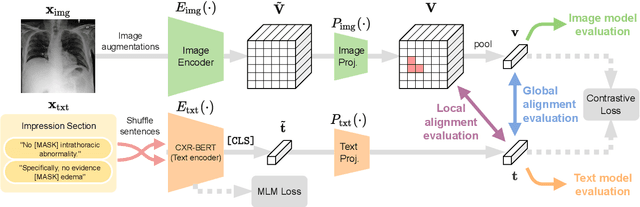
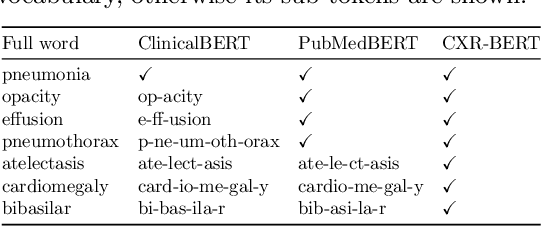
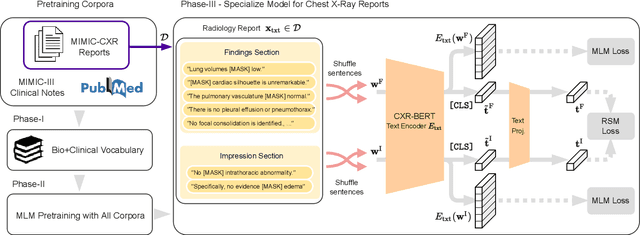
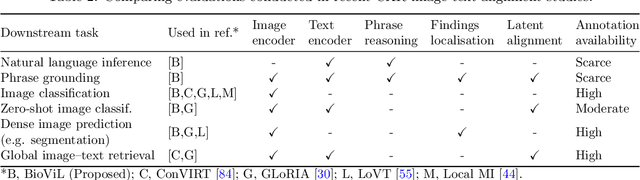
Abstract:Multi-modal data abounds in biomedicine, such as radiology images and reports. Interpreting this data at scale is essential for improving clinical care and accelerating clinical research. Biomedical text with its complex semantics poses additional challenges in vision-language modelling compared to the general domain, and previous work has used insufficiently adapted models that lack domain-specific language understanding. In this paper, we show that principled textual semantic modelling can substantially improve contrastive learning in self-supervised vision--language processing. We release a language model that achieves state-of-the-art results in radiology natural language inference through its improved vocabulary and novel language pretraining objective leveraging semantics and discourse characteristics in radiology reports. Further, we propose a self-supervised joint vision--language approach with a focus on better text modelling. It establishes new state of the art results on a wide range of publicly available benchmarks, in part by leveraging our new domain-specific language model. We release a new dataset with locally-aligned phrase grounding annotations by radiologists to facilitate the study of complex semantic modelling in biomedical vision--language processing. A broad evaluation, including on this new dataset, shows that our contrastive learning approach, aided by textual-semantic modelling, outperforms prior methods in segmentation tasks, despite only using a global-alignment objective.
Towards Structuring Real-World Data at Scale: Deep Learning for Extracting Key Oncology Information from Clinical Text with Patient-Level Supervision
Mar 20, 2022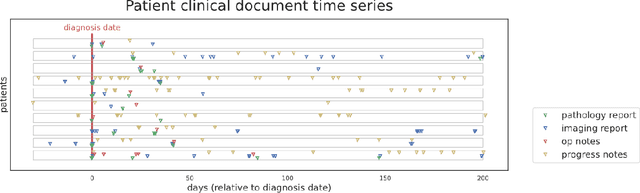
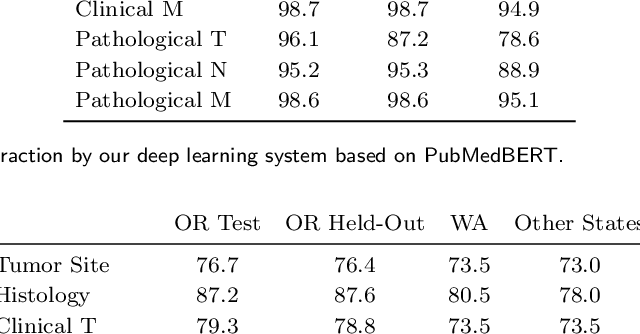
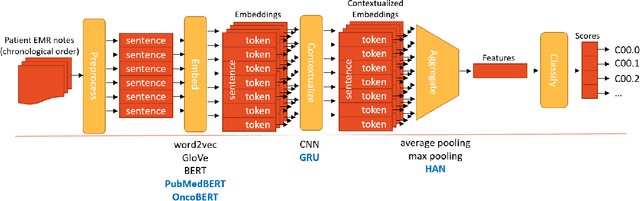
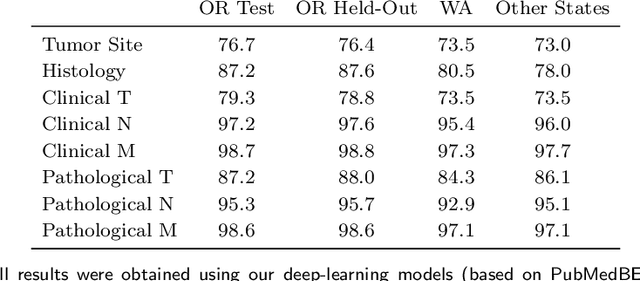
Abstract:Objective: The majority of detailed patient information in real-world data (RWD) is only consistently available in free-text clinical documents. Manual curation is expensive and time-consuming. Developing natural language processing (NLP) methods for structuring RWD is thus essential for scaling real-world evidence generation. Materials and Methods: Traditional rule-based systems are vulnerable to the prevalent linguistic variations and ambiguities in clinical text, and prior applications of machine-learning methods typically require sentence-level or report-level labeled examples that are hard to produce at scale. We propose leveraging patient-level supervision from medical registries, which are often readily available and capture key patient information, for general RWD applications. To combat the lack of sentence-level or report-level annotations, we explore advanced deep-learning methods by combining domain-specific pretraining, recurrent neural networks, and hierarchical attention. Results: We conduct an extensive study on 135,107 patients from the cancer registry of a large integrated delivery network (IDN) comprising healthcare systems in five western US states. Our deep learning methods attain test AUROC of 94-99% for key tumor attributes and comparable performance on held-out data from separate health systems and states. Discussion and Conclusion: Ablation results demonstrate clear superiority of these advanced deep-learning methods over prior approaches. Error analysis shows that our NLP system sometimes even corrects errors in registrar labels. We also conduct a preliminary investigation in accelerating registry curation and general RWD structuring via assisted curation for over 1.2 million cancer patients in this healthcare network.
Knowledge-Rich Self-Supervised Entity Linking
Dec 15, 2021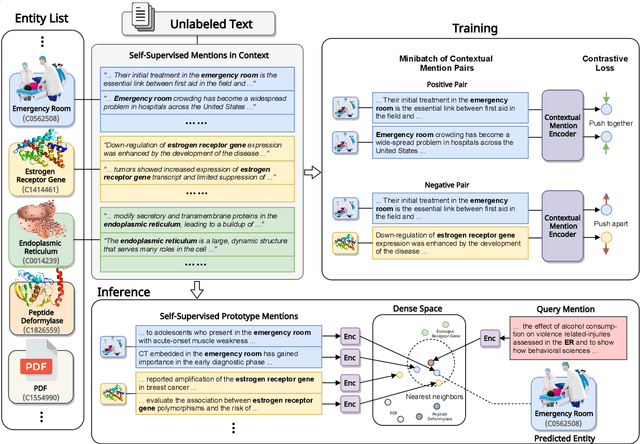
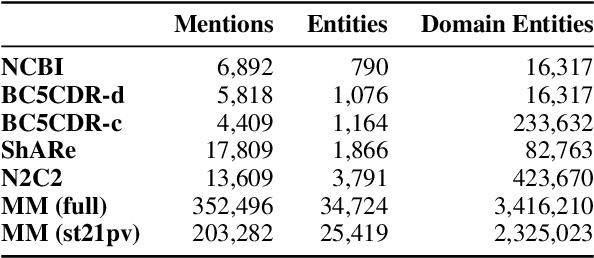
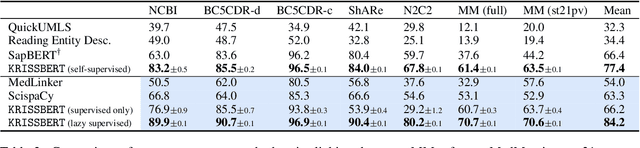
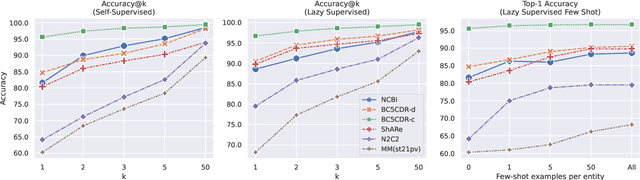
Abstract:Entity linking faces significant challenges, such as prolific variations and prevalent ambiguities, especially in high-value domains with myriad entities. Standard classification approaches suffer from the annotation bottleneck and cannot effectively handle unseen entities. Zero-shot entity linking has emerged as a promising direction for generalizing to new entities, but it still requires example gold entity mentions during training and canonical descriptions for all entities, both of which are rarely available outside of Wikipedia. In this paper, we explore Knowledge-RIch Self-Supervision ($\tt KRISS$) for entity linking, by leveraging readily available domain knowledge. In training, it generates self-supervised mention examples on unlabeled text using a domain ontology and trains a contextual encoder using contrastive learning. For inference, it samples self-supervised mentions as prototypes for each entity and conducts linking by mapping the test mention to the most similar prototype. Our approach subsumes zero-shot and few-shot methods, and can easily incorporate entity descriptions and gold mention labels if available. Using biomedicine as a case study, we conducted extensive experiments on seven standard datasets spanning biomedical literature and clinical notes. Without using any labeled information, our method produces $\tt KRISSBERT$, a universal entity linker for four million UMLS entities, which attains new state of the art, outperforming prior self-supervised methods by as much as over 20 absolute points in accuracy.
Fine-Tuning Large Neural Language Models for Biomedical Natural Language Processing
Dec 15, 2021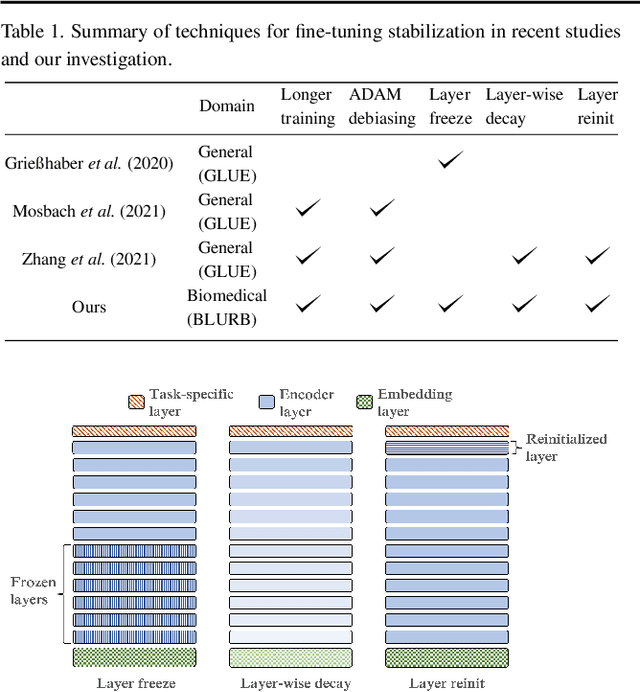
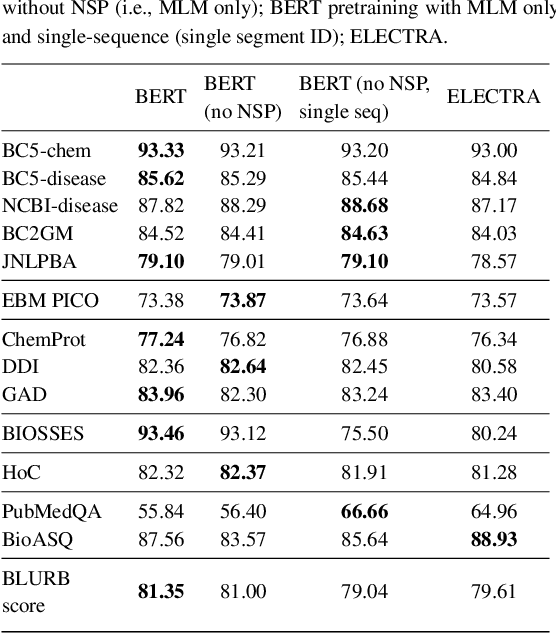
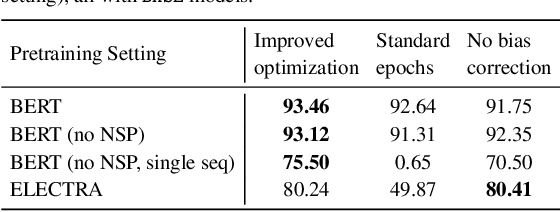
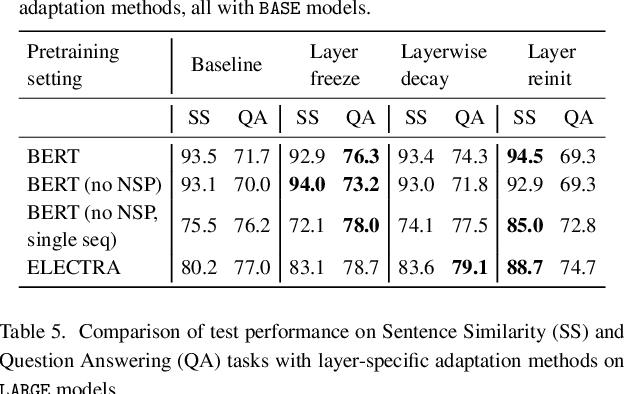
Abstract:Motivation: A perennial challenge for biomedical researchers and clinical practitioners is to stay abreast with the rapid growth of publications and medical notes. Natural language processing (NLP) has emerged as a promising direction for taming information overload. In particular, large neural language models facilitate transfer learning by pretraining on unlabeled text, as exemplified by the successes of BERT models in various NLP applications. However, fine-tuning such models for an end task remains challenging, especially with small labeled datasets, which are common in biomedical NLP. Results: We conduct a systematic study on fine-tuning stability in biomedical NLP. We show that finetuning performance may be sensitive to pretraining settings, especially in low-resource domains. Large models have potential to attain better performance, but increasing model size also exacerbates finetuning instability. We thus conduct a comprehensive exploration of techniques for addressing fine-tuning instability. We show that these techniques can substantially improve fine-tuning performance for lowresource biomedical NLP applications. Specifically, freezing lower layers is helpful for standard BERT-BASE models, while layerwise decay is more effective for BERT-LARGE and ELECTRA models. For low-resource text similarity tasks such as BIOSSES, reinitializing the top layer is the optimal strategy. Overall, domainspecific vocabulary and pretraining facilitate more robust models for fine-tuning. Based on these findings, we establish new state of the art on a wide range of biomedical NLP applications. Availability and implementation: To facilitate progress in biomedical NLP, we release our state-of-the-art pretrained and fine-tuned models: https://aka.ms/BLURB.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge