Umesh Telang
Plots Unlock Time-Series Understanding in Multimodal Models
Oct 03, 2024



Abstract:While multimodal foundation models can now natively work with data beyond text, they remain underutilized in analyzing the considerable amounts of multi-dimensional time-series data in fields like healthcare, finance, and social sciences, representing a missed opportunity for richer, data-driven insights. This paper proposes a simple but effective method that leverages the existing vision encoders of these models to "see" time-series data via plots, avoiding the need for additional, potentially costly, model training. Our empirical evaluations show that this approach outperforms providing the raw time-series data as text, with the additional benefit that visual time-series representations demonstrate up to a 90% reduction in model API costs. We validate our hypothesis through synthetic data tasks of increasing complexity, progressing from simple functional form identification on clean data, to extracting trends from noisy scatter plots. To demonstrate generalizability from synthetic tasks with clear reasoning steps to more complex, real-world scenarios, we apply our approach to consumer health tasks - specifically fall detection, activity recognition, and readiness assessment - which involve heterogeneous, noisy data and multi-step reasoning. The overall success in plot performance over text performance (up to an 120% performance increase on zero-shot synthetic tasks, and up to 150% performance increase on real-world tasks), across both GPT and Gemini model families, highlights our approach's potential for making the best use of the native capabilities of foundation models.
MINT: A wrapper to make multi-modal and multi-image AI models interactive
Jan 22, 2024Abstract:During the diagnostic process, doctors incorporate multimodal information including imaging and the medical history - and similarly medical AI development has increasingly become multimodal. In this paper we tackle a more subtle challenge: doctors take a targeted medical history to obtain only the most pertinent pieces of information; how do we enable AI to do the same? We develop a wrapper method named MINT (Make your model INTeractive) that automatically determines what pieces of information are most valuable at each step, and ask for only the most useful information. We demonstrate the efficacy of MINT wrapping a skin disease prediction model, where multiple images and a set of optional answers to $25$ standard metadata questions (i.e., structured medical history) are used by a multi-modal deep network to provide a differential diagnosis. We show that MINT can identify whether metadata inputs are needed and if so, which question to ask next. We also demonstrate that when collecting multiple images, MINT can identify if an additional image would be beneficial, and if so, which type of image to capture. We showed that MINT reduces the number of metadata and image inputs needed by 82% and 36.2% respectively, while maintaining predictive performance. Using real-world AI dermatology system data, we show that needing fewer inputs can retain users that may otherwise fail to complete the system submission and drop off without a diagnosis. Qualitative examples show MINT can closely mimic the step-by-step decision making process of a clinical workflow and how this is different for straight forward cases versus more difficult, ambiguous cases. Finally we demonstrate how MINT is robust to different underlying multi-model classifiers and can be easily adapted to user requirements without significant model re-training.
Evaluating AI systems under uncertain ground truth: a case study in dermatology
Jul 05, 2023



Abstract:For safety, AI systems in health undergo thorough evaluations before deployment, validating their predictions against a ground truth that is assumed certain. However, this is actually not the case and the ground truth may be uncertain. Unfortunately, this is largely ignored in standard evaluation of AI models but can have severe consequences such as overestimating the future performance. To avoid this, we measure the effects of ground truth uncertainty, which we assume decomposes into two main components: annotation uncertainty which stems from the lack of reliable annotations, and inherent uncertainty due to limited observational information. This ground truth uncertainty is ignored when estimating the ground truth by deterministically aggregating annotations, e.g., by majority voting or averaging. In contrast, we propose a framework where aggregation is done using a statistical model. Specifically, we frame aggregation of annotations as posterior inference of so-called plausibilities, representing distributions over classes in a classification setting, subject to a hyper-parameter encoding annotator reliability. Based on this model, we propose a metric for measuring annotation uncertainty and provide uncertainty-adjusted metrics for performance evaluation. We present a case study applying our framework to skin condition classification from images where annotations are provided in the form of differential diagnoses. The deterministic adjudication process called inverse rank normalization (IRN) from previous work ignores ground truth uncertainty in evaluation. Instead, we present two alternative statistical models: a probabilistic version of IRN and a Plackett-Luce-based model. We find that a large portion of the dataset exhibits significant ground truth uncertainty and standard IRN-based evaluation severely over-estimates performance without providing uncertainty estimates.
Robust and Efficient Medical Imaging with Self-Supervision
May 19, 2022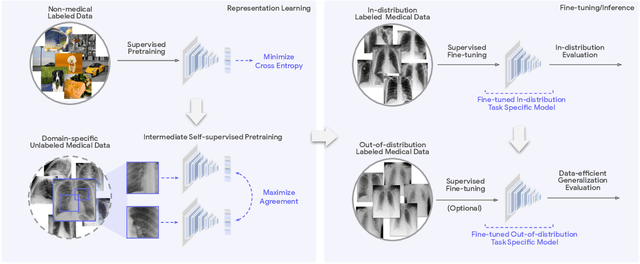
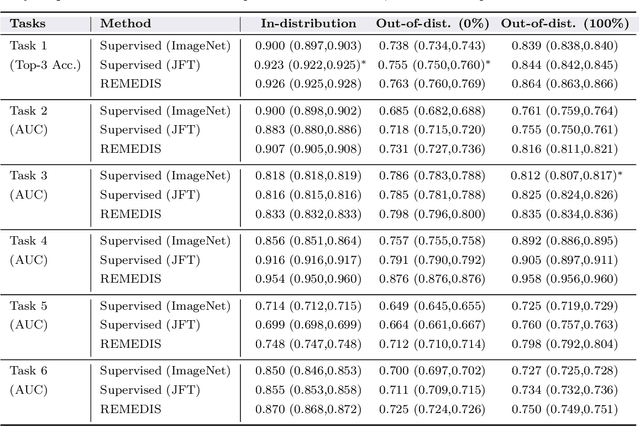
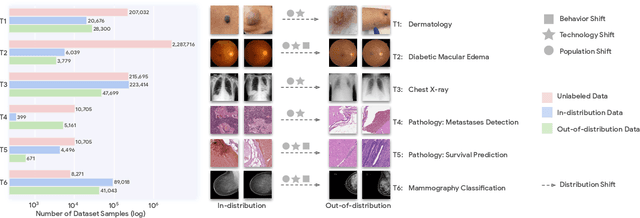

Abstract:Recent progress in Medical Artificial Intelligence (AI) has delivered systems that can reach clinical expert level performance. However, such systems tend to demonstrate sub-optimal "out-of-distribution" performance when evaluated in clinical settings different from the training environment. A common mitigation strategy is to develop separate systems for each clinical setting using site-specific data [1]. However, this quickly becomes impractical as medical data is time-consuming to acquire and expensive to annotate [2]. Thus, the problem of "data-efficient generalization" presents an ongoing difficulty for Medical AI development. Although progress in representation learning shows promise, their benefits have not been rigorously studied, specifically for out-of-distribution settings. To meet these challenges, we present REMEDIS, a unified representation learning strategy to improve robustness and data-efficiency of medical imaging AI. REMEDIS uses a generic combination of large-scale supervised transfer learning with self-supervised learning and requires little task-specific customization. We study a diverse range of medical imaging tasks and simulate three realistic application scenarios using retrospective data. REMEDIS exhibits significantly improved in-distribution performance with up to 11.5% relative improvement in diagnostic accuracy over a strong supervised baseline. More importantly, our strategy leads to strong data-efficient generalization of medical imaging AI, matching strong supervised baselines using between 1% to 33% of retraining data across tasks. These results suggest that REMEDIS can significantly accelerate the life-cycle of medical imaging AI development thereby presenting an important step forward for medical imaging AI to deliver broad impact.
Does Your Dermatology Classifier Know What It Doesn't Know? Detecting the Long-Tail of Unseen Conditions
Apr 08, 2021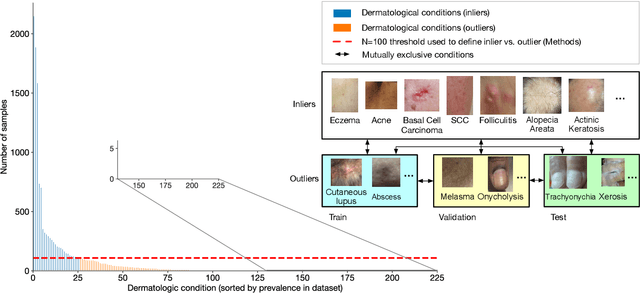
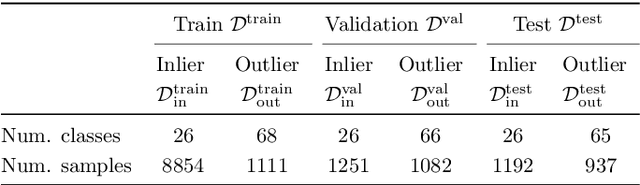
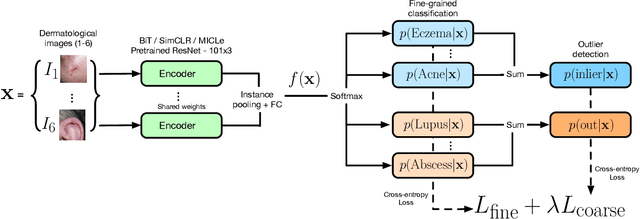
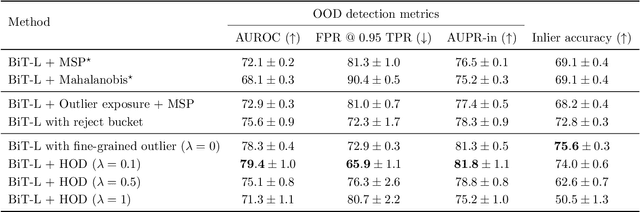
Abstract:We develop and rigorously evaluate a deep learning based system that can accurately classify skin conditions while detecting rare conditions for which there is not enough data available for training a confident classifier. We frame this task as an out-of-distribution (OOD) detection problem. Our novel approach, hierarchical outlier detection (HOD) assigns multiple abstention classes for each training outlier class and jointly performs a coarse classification of inliers vs. outliers, along with fine-grained classification of the individual classes. We demonstrate the effectiveness of the HOD loss in conjunction with modern representation learning approaches (BiT, SimCLR, MICLe) and explore different ensembling strategies for further improving the results. We perform an extensive subgroup analysis over conditions of varying risk levels and different skin types to investigate how the OOD detection performance changes over each subgroup and demonstrate the gains of our framework in comparison to baselines. Finally, we introduce a cost metric to approximate downstream clinical impact. We use this cost metric to compare the proposed method against a baseline system, thereby making a stronger case for the overall system effectiveness in a real-world deployment scenario.
Supervised Transfer Learning at Scale for Medical Imaging
Jan 21, 2021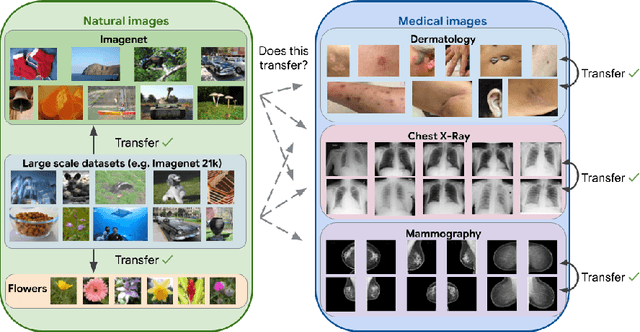
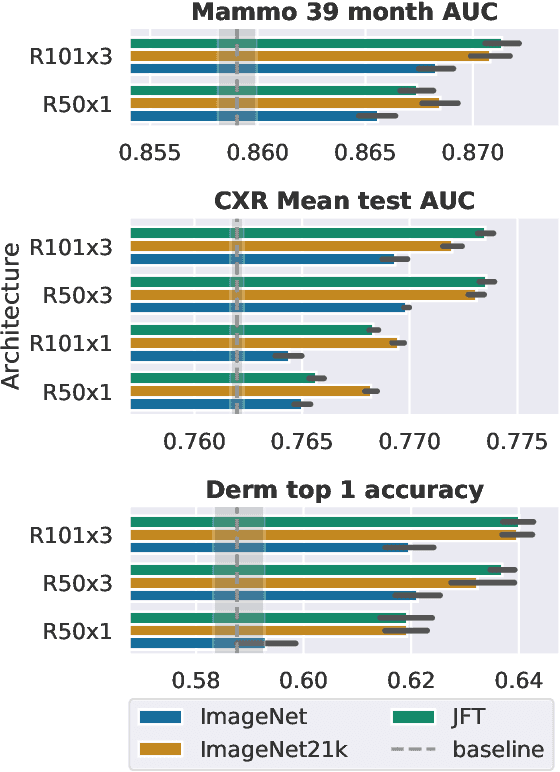
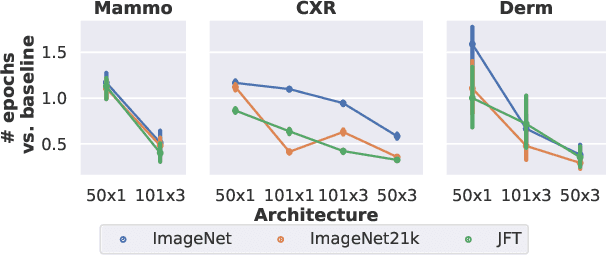
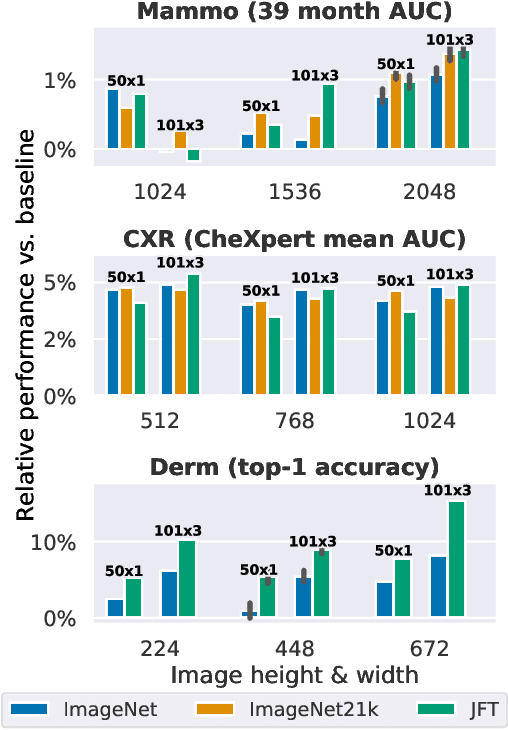
Abstract:Transfer learning is a standard technique to improve performance on tasks with limited data. However, for medical imaging, the value of transfer learning is less clear. This is likely due to the large domain mismatch between the usual natural-image pre-training (e.g. ImageNet) and medical images. However, recent advances in transfer learning have shown substantial improvements from scale. We investigate whether modern methods can change the fortune of transfer learning for medical imaging. For this, we study the class of large-scale pre-trained networks presented by Kolesnikov et al. on three diverse imaging tasks: chest radiography, mammography, and dermatology. We study both transfer performance and critical properties for the deployment in the medical domain, including: out-of-distribution generalization, data-efficiency, sub-group fairness, and uncertainty estimation. Interestingly, we find that for some of these properties transfer from natural to medical images is indeed extremely effective, but only when performed at sufficient scale.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge