Jingsong Li
Integrating clinical reasoning into large language model-based diagnosis through etiology-aware attention steering
Aug 01, 2025Abstract:Objective: Large Language Models (LLMs) demonstrate significant capabilities in medical text understanding and generation. However, their diagnostic reliability in complex clinical scenarios remains limited. This study aims to enhance LLMs' diagnostic accuracy and clinical reasoning ability. Method: We propose an Etiology-Aware Attention Steering Framework to integrate structured clinical reasoning into LLM-based diagnosis. Specifically, we first construct Clinical Reasoning Scaffolding (CRS) based on authoritative clinical guidelines for three representative acute abdominal emergencies: acute appendicitis, acute pancreatitis, and acute cholecystitis. Next, we develop the Etiology-Aware Head Identification algorithm to pinpoint attention heads crucial for the model's etiology reasoning. To ensure reliable clinical reasoning alignment, we introduce the Reasoning-Guided Parameter-Efficient Fine-tuning that embeds etiological reasoning cues into input representations and steers the selected Etiology-Aware Heads toward critical information through a Reasoning-Guided Loss function. Result: On the Consistent Diagnosis Cohort, our framework improves average diagnostic accuracy by 15.65% and boosts the average Reasoning Focus Score by 31.6% over baselines. External validation on the Discrepant Diagnosis Cohort further confirms its effectiveness in enhancing diagnostic accuracy. Further assessments via Reasoning Attention Frequency indicate that our models exhibit enhanced reliability when faced with real-world complex scenarios. Conclusion: This study presents a practical and effective approach to enhance clinical reasoning in LLM-based diagnosis. By aligning model attention with structured CRS, the proposed framework offers a promising paradigm for building more interpretable and reliable AI diagnostic systems in complex clinical settings.
From Questions to Clinical Recommendations: Large Language Models Driving Evidence-Based Clinical Decision Making
May 15, 2025



Abstract:Clinical evidence, derived from rigorous research and data analysis, provides healthcare professionals with reliable scientific foundations for informed decision-making. Integrating clinical evidence into real-time practice is challenging due to the enormous workload, complex professional processes, and time constraints. This highlights the need for tools that automate evidence synthesis to support more efficient and accurate decision making in clinical settings. This study introduces Quicker, an evidence-based clinical decision support system powered by large language models (LLMs), designed to automate evidence synthesis and generate clinical recommendations modeled after standard clinical guideline development processes. Quicker implements a fully automated chain that covers all phases, from questions to clinical recommendations, and further enables customized decision-making through integrated tools and interactive user interfaces. To evaluate Quicker's capabilities, we developed the Q2CRBench-3 benchmark dataset, based on clinical guideline development records for three different diseases. Experimental results highlighted Quicker's strong performance, with fine-grained question decomposition tailored to user preferences, retrieval sensitivities comparable to human experts, and literature screening performance approaching comprehensive inclusion of relevant studies. In addition, Quicker-assisted evidence assessment effectively supported human reviewers, while Quicker's recommendations were more comprehensive and logically coherent than those of clinicians. In system-level testing, collaboration between a single reviewer and Quicker reduced the time required for recommendation development to 20-40 minutes. In general, our findings affirm the potential of Quicker to help physicians make quicker and more reliable evidence-based clinical decisions.
Generating Synthetic Mixed-type Longitudinal Electronic Health Records for Artificial Intelligent Applications
Dec 22, 2021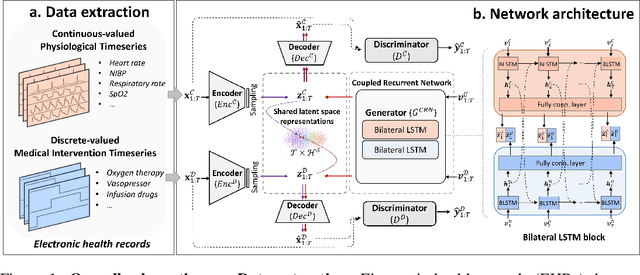

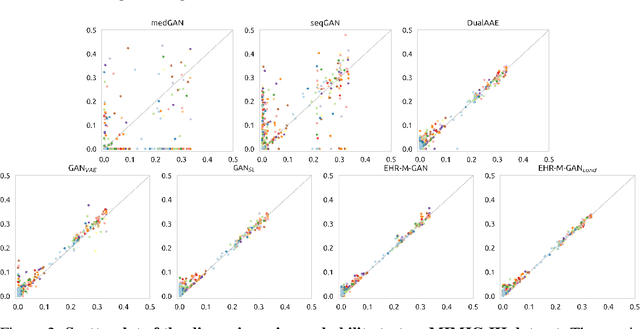
Abstract:The recent availability of electronic health records (EHRs) have provided enormous opportunities to develop artificial intelligence (AI) algorithms. However, patient privacy has become a major concern that limits data sharing across hospital settings and subsequently hinders the advances in AI. \textit{Synthetic data}, which benefits from the development and proliferation of generative models, has served as a promising substitute for real patient EHR data. However, the current generative models are limited as they only generate \textit{single type} of clinical data, i.e., either continuous-valued or discrete-valued. In this paper, we propose a generative adversarial network (GAN) entitled EHR-M-GAN which synthesizes \textit{mixed-type} timeseries EHR data. EHR-M-GAN is capable of capturing the multidimensional, heterogeneous, and correlated temporal dynamics in patient trajectories. We have validated EHR-M-GAN on three publicly-available intensive care unit databases with records from a total of 141,488 unique patients, and performed privacy risk evaluation of the proposed model. EHR-M-GAN has demonstrated its superiority in performance over state-of-the-art benchmarks for synthesizing clinical timeseries with high fidelity. Notably, prediction models for outcomes of intensive care performed significantly better when training data was augmented with the addition of EHR-M-GAN-generated timeseries. EHR-M-GAN may have use in developing AI algorithms in resource-limited settings, lowering the barrier for data acquisition while preserving patient privacy.
Multi-phase Liver Tumor Segmentation with Spatial Aggregation and Uncertain Region Inpainting
Aug 05, 2021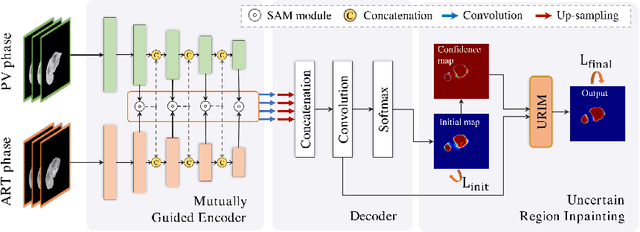
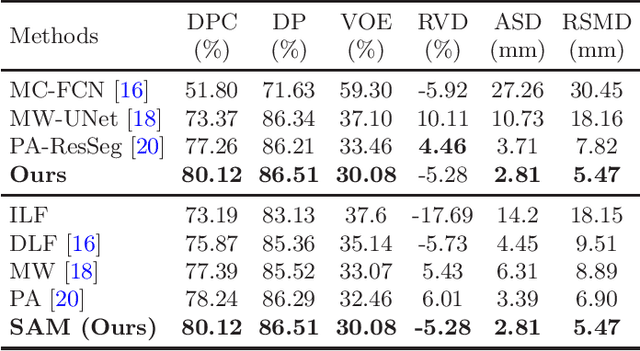
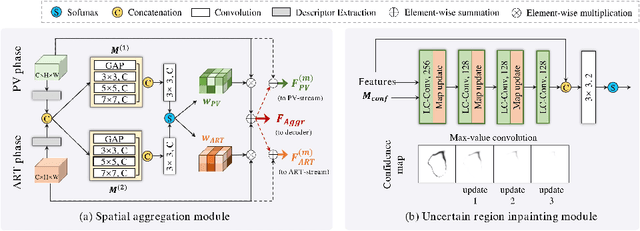
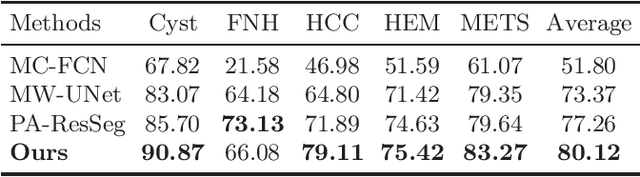
Abstract:Multi-phase computed tomography (CT) images provide crucial complementary information for accurate liver tumor segmentation (LiTS). State-of-the-art multi-phase LiTS methods usually fused cross-phase features through phase-weighted summation or channel-attention based concatenation. However, these methods ignored the spatial (pixel-wise) relationships between different phases, hence leading to insufficient feature integration. In addition, the performance of existing methods remains subject to the uncertainty in segmentation, which is particularly acute in tumor boundary regions. In this work, we propose a novel LiTS method to adequately aggregate multi-phase information and refine uncertain region segmentation. To this end, we introduce a spatial aggregation module (SAM), which encourages per-pixel interactions between different phases, to make full use of cross-phase information. Moreover, we devise an uncertain region inpainting module (URIM) to refine uncertain pixels using neighboring discriminative features. Experiments on an in-house multi-phase CT dataset of focal liver lesions (MPCT-FLLs) demonstrate that our method achieves promising liver tumor segmentation and outperforms state-of-the-arts.
Interactive Deep Refinement Network for Medical Image Segmentation
Jun 27, 2020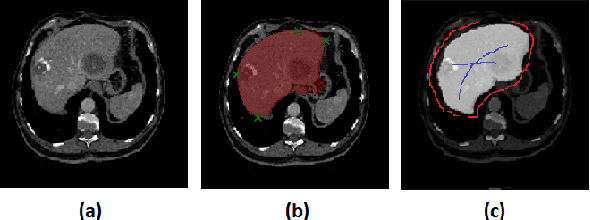
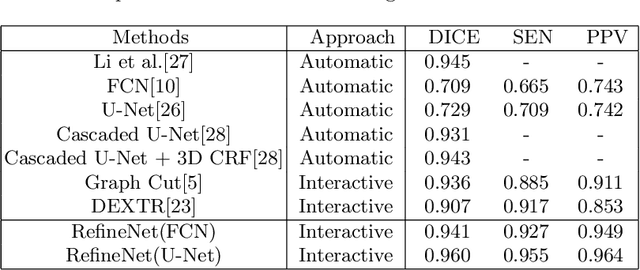
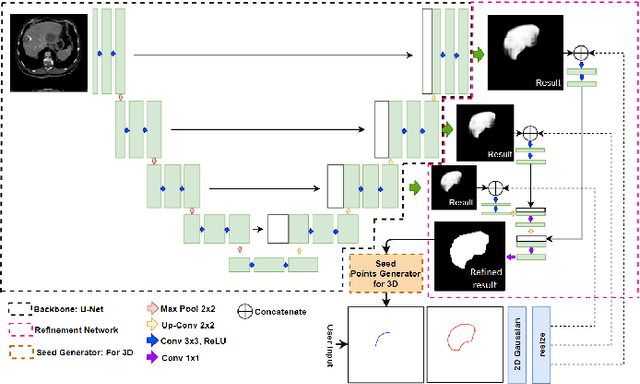
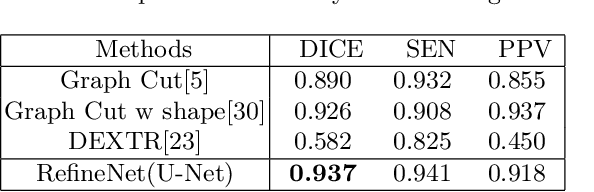
Abstract:Deep learning techniques have successfully been employed in numerous computer vision tasks including image segmentation. The techniques have also been applied to medical image segmentation, one of the most critical tasks in computer-aided diagnosis. Compared with natural images, the medical image is a gray-scale image with low-contrast (even with some invisible parts). Because some organs have similar intensity and texture with neighboring organs, there is usually a need to refine automatic segmentation results. In this paper, we propose an interactive deep refinement framework to improve the traditional semantic segmentation networks such as U-Net and fully convolutional network. In the proposed framework, we added a refinement network to traditional segmentation network to refine the segmentation results.Experimental results with public dataset revealed that the proposed method could achieve higher accuracy than other state-of-the-art methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge