J. Alison Noble
Self-supervised Representation Learning for Ultrasound Video
Feb 28, 2020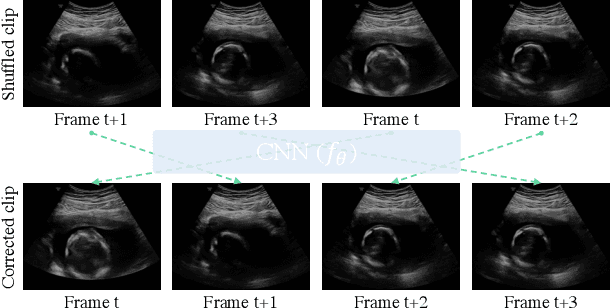
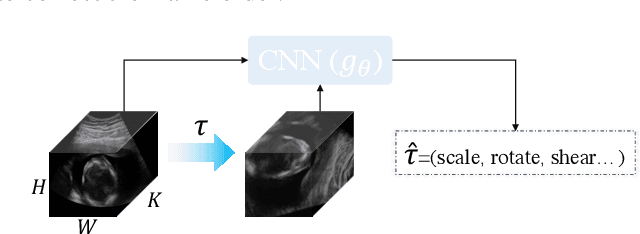
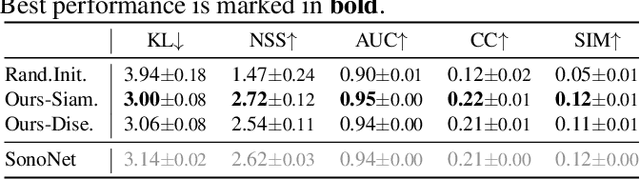
Abstract:Recent advances in deep learning have achieved promising performance for medical image analysis, while in most cases ground-truth annotations from human experts are necessary to train the deep model. In practice, such annotations are expensive to collect and can be scarce for medical imaging applications. Therefore, there is significant interest in learning representations from unlabelled raw data. In this paper, we propose a self-supervised learning approach to learn meaningful and transferable representations from medical imaging video without any type of human annotation. We assume that in order to learn such a representation, the model should identify anatomical structures from the unlabelled data. Therefore we force the model to address anatomy-aware tasks with free supervision from the data itself. Specifically, the model is designed to correct the order of a reshuffled video clip and at the same time predict the geometric transformation applied to the video clip. Experiments on fetal ultrasound video show that the proposed approach can effectively learn meaningful and strong representations, which transfer well to downstream tasks like standard plane detection and saliency prediction.
Discovering Salient Anatomical Landmarks by Predicting Human Gaze
Jan 22, 2020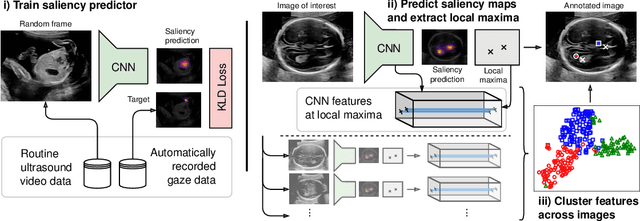
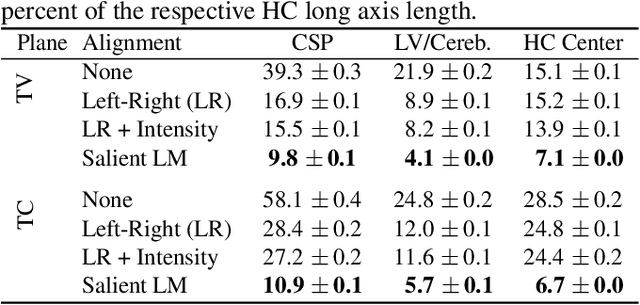
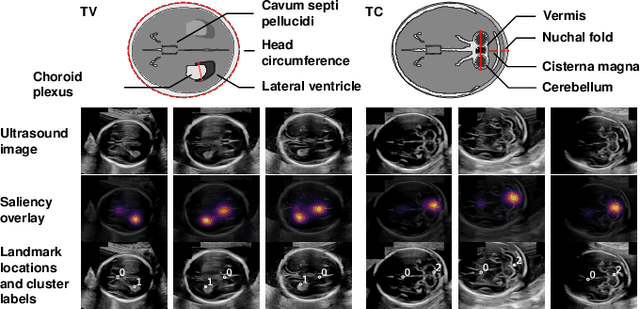
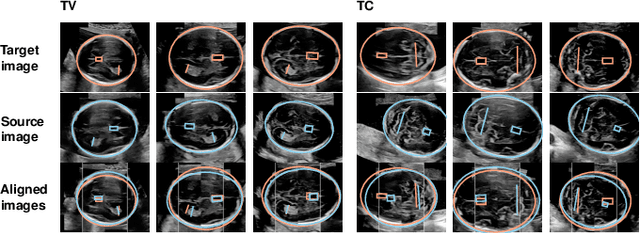
Abstract:Anatomical landmarks are a crucial prerequisite for many medical imaging tasks. Usually, the set of landmarks for a given task is predefined by experts. The landmark locations for a given image are then annotated manually or via machine learning methods trained on manual annotations. In this paper, in contrast, we present a method to automatically discover and localize anatomical landmarks in medical images. Specifically, we consider landmarks that attract the visual attention of humans, which we term visually salient landmarks. We illustrate the method for fetal neurosonographic images. First, full-length clinical fetal ultrasound scans are recorded with live sonographer gaze-tracking. Next, a convolutional neural network (CNN) is trained to predict the gaze point distribution (saliency map) of the sonographers on scan video frames. The CNN is then used to predict saliency maps of unseen fetal neurosonographic images, and the landmarks are extracted as the local maxima of these saliency maps. Finally, the landmarks are matched across images by clustering the landmark CNN features. We show that the discovered landmarks can be used within affine image registration, with average landmark alignment errors between 4.1% and 10.9% of the fetal head long axis length.
Heterogeneous tissue characterization using ultrasound: a comparison of fractal analysis backscatter models on liver tumors
Dec 20, 2019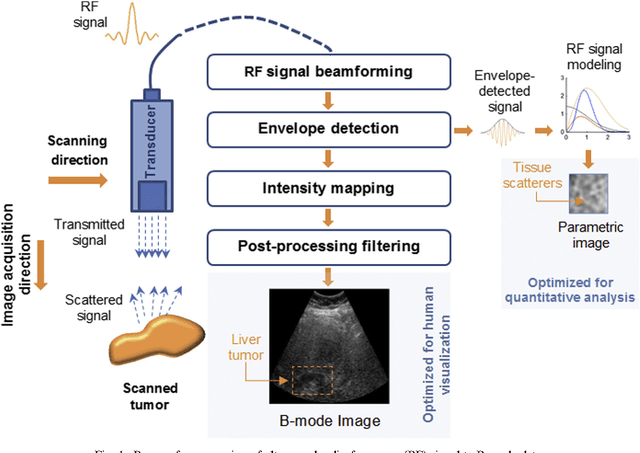
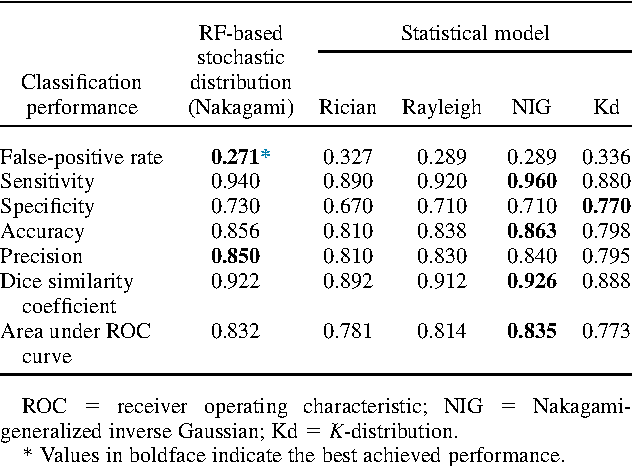
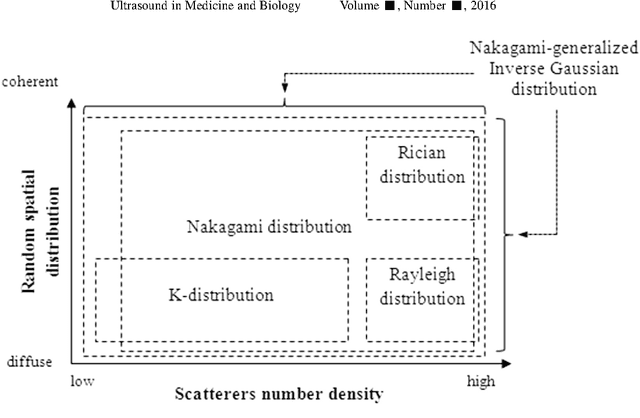
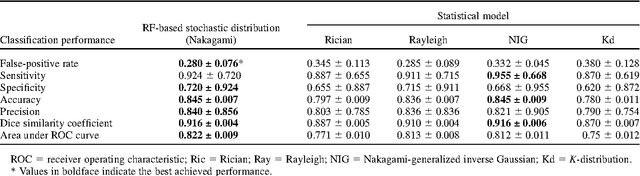
Abstract:Assessing tumor tissue heterogeneity via ultrasound has recently been suggested for predicting early response to treatment. The ultrasound backscattering characteristics can assist in better understanding the tumor texture by highlighting local concentration and spatial arrangement of tissue scatterers. However, it is challenging to quantify the various tissue heterogeneities ranging from fine-to-coarse of the echo envelope peaks in tumor texture. Local parametric fractal features extracted via maximum likelihood estimation from five well-known statistical model families are evaluated for the purpose of ultrasound tissue characterization. The fractal dimension (self-similarity measure) was used to characterize the spatial distribution of scatterers, while the Lacunarity (sparsity measure) was applied to determine scatterer number density. Performance was assessed based on 608 cross-sectional clinical ultrasound RF images of liver tumors (230 and 378 demonstrating respondent and non-respondent cases, respectively). Crossvalidation via leave-one-tumor-out and with different k-folds methodologies using a Bayesian classifier were employed for validation. The fractal properties of the backscattered echoes based on the Nakagami model (Nkg) and its extend four-parameter Nakagami-generalized inverse Gaussian (NIG) distribution achieved best results - with nearly similar performance - for characterizing liver tumor tissue. Accuracy, sensitivity and specificity for the Nkg/NIG were: 85.6%/86.3%, 94.0%/96.0%, and 73.0%/71.0%, respectively. Other statistical models, such as the Rician, Rayleigh, and K-distribution were found to not be as effective in characterizing the subtle changes in tissue texture as an indication of response to treatment. Employing the most relevant and practical statistical model could have potential consequences for the design of an early and effective clinical therapy.
* 31 pages, 7 figures, 3 tables, journal article
UPI-Net: Semantic Contour Detection in Placental Ultrasound
Sep 13, 2019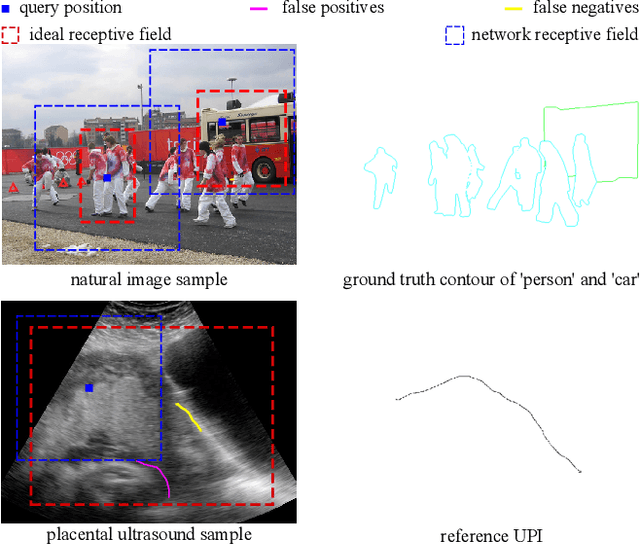
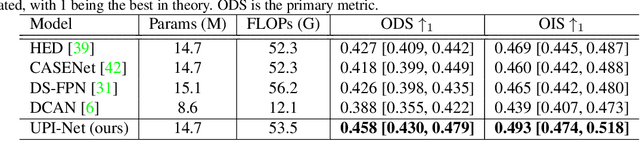
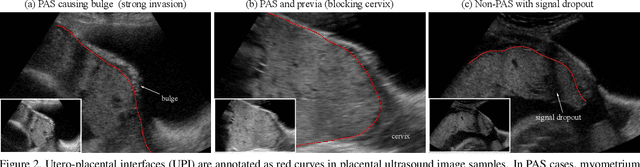
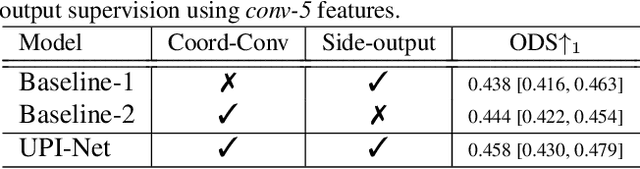
Abstract:Semantic contour detection is a challenging problem that is often met in medical imaging, of which placental image analysis is a particular example. In this paper, we investigate utero-placental interface (UPI) detection in 2D placental ultrasound images by formulating it as a semantic contour detection problem. As opposed to natural images, placental ultrasound images contain specific anatomical structures thus have unique geometry. We argue it would be beneficial for UPI detectors to incorporate global context modelling in order to reduce unwanted false positive UPI predictions. Our approach, namely UPI-Net, aims to capture long-range dependencies in placenta geometry through lightweight global context modelling and effective multi-scale feature aggregation. We perform a subject-level 10-fold nested cross-validation on a placental ultrasound database (4,871 images with labelled UPI from 49 scans). Experimental results demonstrate that, without introducing considerable computational overhead, UPI-Net yields the highest performance in terms of standard contour detection metrics, compared to other competitive benchmarks.
Anatomy-Aware Self-supervised Fetal MRI Synthesis from Unpaired Ultrasound Images
Sep 08, 2019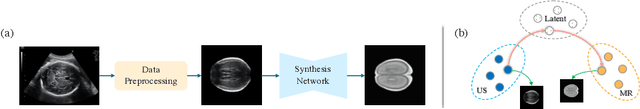
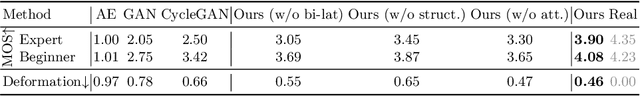
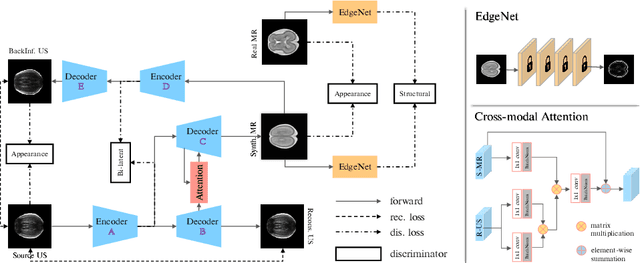
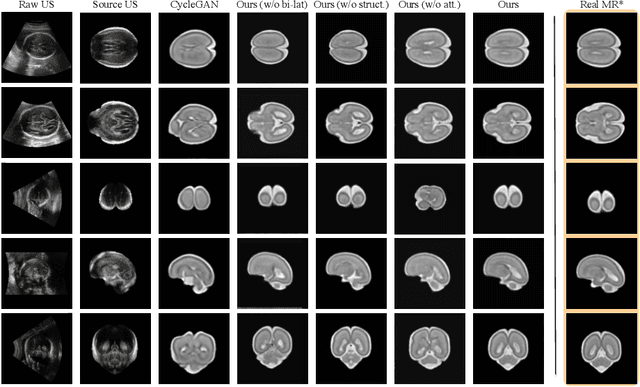
Abstract:Fetal brain magnetic resonance imaging (MRI) offers exquisite images of the developing brain but is not suitable for anomaly screening. For this ultrasound (US) is employed. While expert sonographers are adept at reading US images, MR images are much easier for non-experts to interpret. Hence in this paper we seek to produce images with MRI-like appearance directly from clinical US images. Our own clinical motivation is to seek a way to communicate US findings to patients or clinical professionals unfamiliar with US, but in medical image analysis such a capability is potentially useful, for instance, for US-MRI registration or fusion. Our model is self-supervised and end-to-end trainable. Specifically, based on an assumption that the US and MRI data share a similar anatomical latent space, we first utilise an extractor to determine shared latent features, which are then used for data synthesis. Since paired data was unavailable for our study (and rare in practice), we propose to enforce the distributions to be similar instead of employing pixel-wise constraints, by adversarial learning in both the image domain and latent space. Furthermore, we propose an adversarial structural constraint to regularise the anatomical structures between the two modalities during the synthesis. A cross-modal attention scheme is proposed to leverage non-local spatial correlations. The feasibility of the approach to produce realistic looking MR images is demonstrated quantitatively and with a qualitative evaluation compared to real fetal MR images.
Conditional Segmentation in Lieu of Image Registration
Jun 30, 2019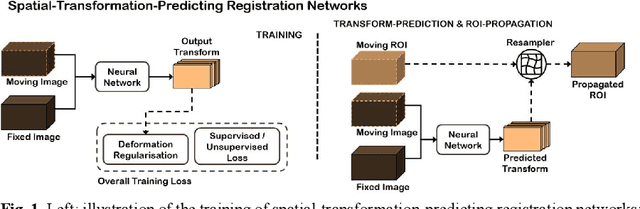
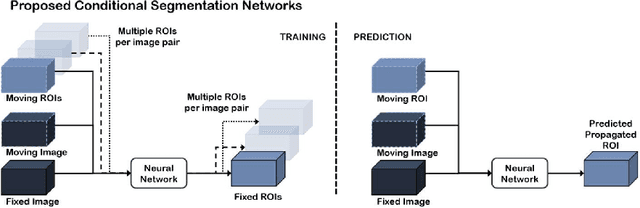
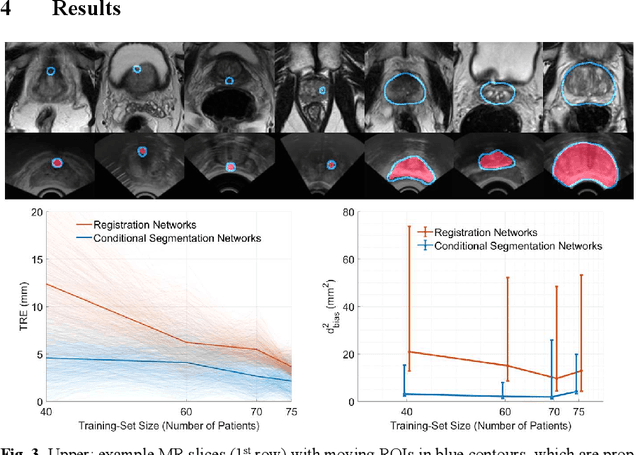
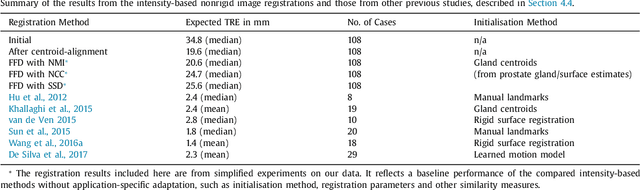
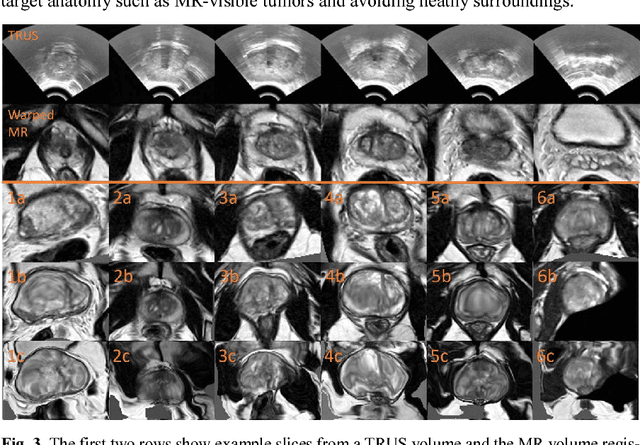
Abstract:Classical pairwise image registration methods search for a spatial transformation that optimises a numerical measure that indicates how well a pair of moving and fixed images are aligned. Current learning-based registration methods have adopted the same paradigm and typically predict, for any new input image pair, dense correspondences in the form of a dense displacement field or parameters of a spatial transformation model. However, in many applications of registration, the spatial transformation itself is only required to propagate points or regions of interest (ROIs). In such cases, detailed pixel- or voxel-level correspondence within or outside of these ROIs often have little clinical value. In this paper, we propose an alternative paradigm in which the location of corresponding image-specific ROIs, defined in one image, within another image is learnt. This results in replacing image registration by a conditional segmentation algorithm, which can build on typical image segmentation networks and their widely-adopted training strategies. Using the registration of 3D MRI and ultrasound images of the prostate as an example to demonstrate this new approach, we report a median target registration error (TRE) of 2.1 mm between the ground-truth ROIs defined on intraoperative ultrasound images and those propagated from the preoperative MR images. Significantly lower (>34%) TREs were obtained using the proposed conditional segmentation compared with those obtained from a previously-proposed spatial-transformation-predicting registration network trained with the same multiple ROI labels for individual image pairs. We conclude this work by using a quantitative bias-variance analysis to provide one explanation of the observed improvement in registration accuracy.
Ultrasound Image Representation Learning by Modeling Sonographer Visual Attention
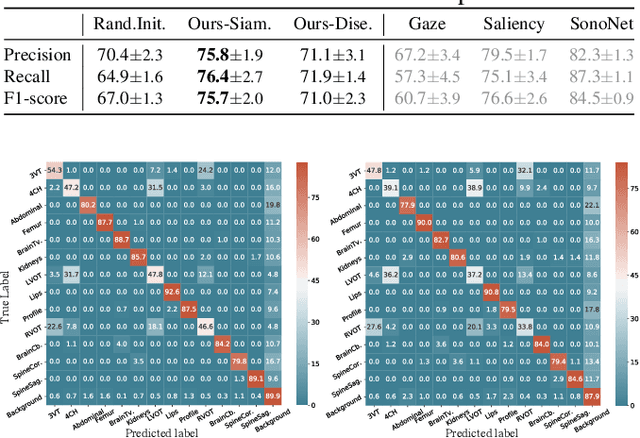
Mar 07, 2019Abstract:Image representations are commonly learned from class labels, which are a simplistic approximation of human image understanding. In this paper we demonstrate that transferable representations of images can be learned without manual annotations by modeling human visual attention. The basis of our analyses is a unique gaze tracking dataset of sonographers performing routine clinical fetal anomaly screenings. Models of sonographer visual attention are learned by training a convolutional neural network (CNN) to predict gaze on ultrasound video frames through visual saliency prediction or gaze-point regression. We evaluate the transferability of the learned representations to the task of ultrasound standard plane detection in two contexts. Firstly, we perform transfer learning by fine-tuning the CNN with a limited number of labeled standard plane images. We find that fine-tuning the saliency predictor is superior to training from random initialization, with an average F1-score improvement of 9.6% overall and 15.3% for the cardiac planes. Secondly, we train a simple softmax regression on the feature activations of each CNN layer in order to evaluate the representations independently of transfer learning hyper-parameters. We find that the attention models derive strong representations, approaching the precision of a fully-supervised baseline model for all but the last layer.
Weakly-Supervised Convolutional Neural Networks for Multimodal Image Registration
Jul 09, 2018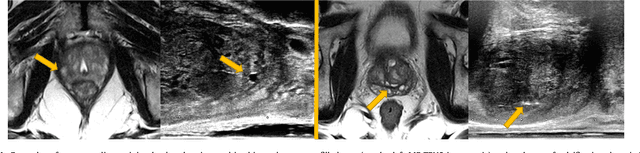
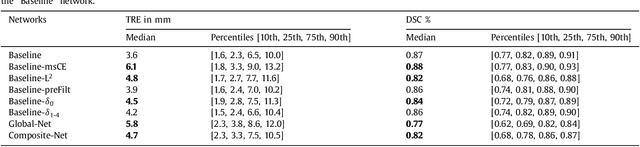
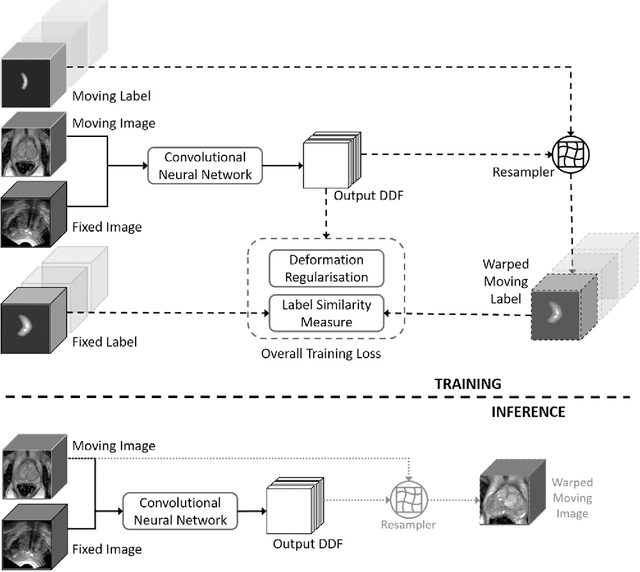
Abstract:One of the fundamental challenges in supervised learning for multimodal image registration is the lack of ground-truth for voxel-level spatial correspondence. This work describes a method to infer voxel-level transformation from higher-level correspondence information contained in anatomical labels. We argue that such labels are more reliable and practical to obtain for reference sets of image pairs than voxel-level correspondence. Typical anatomical labels of interest may include solid organs, vessels, ducts, structure boundaries and other subject-specific ad hoc landmarks. The proposed end-to-end convolutional neural network approach aims to predict displacement fields to align multiple labelled corresponding structures for individual image pairs during the training, while only unlabelled image pairs are used as the network input for inference. We highlight the versatility of the proposed strategy, for training, utilising diverse types of anatomical labels, which need not to be identifiable over all training image pairs. At inference, the resulting 3D deformable image registration algorithm runs in real-time and is fully-automated without requiring any anatomical labels or initialisation. Several network architecture variants are compared for registering T2-weighted magnetic resonance images and 3D transrectal ultrasound images from prostate cancer patients. A median target registration error of 3.6 mm on landmark centroids and a median Dice of 0.87 on prostate glands are achieved from cross-validation experiments, in which 108 pairs of multimodal images from 76 patients were tested with high-quality anatomical labels.
Adversarial Deformation Regularization for Training Image Registration Neural Networks
May 27, 2018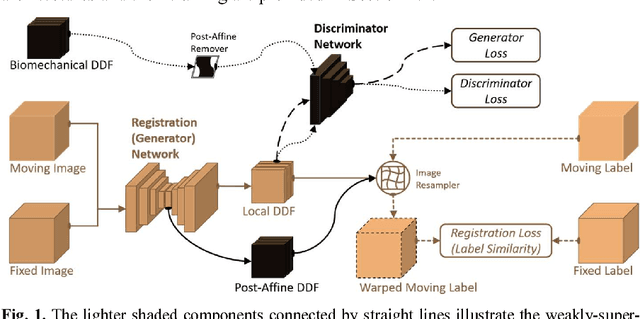
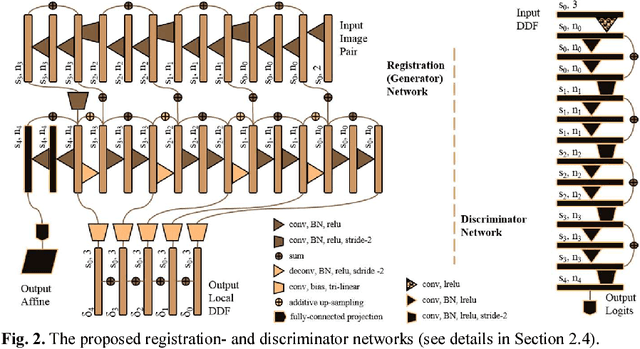
Abstract:We describe an adversarial learning approach to constrain convolutional neural network training for image registration, replacing heuristic smoothness measures of displacement fields often used in these tasks. Using minimally-invasive prostate cancer intervention as an example application, we demonstrate the feasibility of utilizing biomechanical simulations to regularize a weakly-supervised anatomical-label-driven registration network for aligning pre-procedural magnetic resonance (MR) and 3D intra-procedural transrectal ultrasound (TRUS) images. A discriminator network is optimized to distinguish the registration-predicted displacement fields from the motion data simulated by finite element analysis. During training, the registration network simultaneously aims to maximize similarity between anatomical labels that drives image alignment and to minimize an adversarial generator loss that measures divergence between the predicted- and simulated deformation. The end-to-end trained network enables efficient and fully-automated registration that only requires an MR and TRUS image pair as input, without anatomical labels or simulated data during inference. 108 pairs of labelled MR and TRUS images from 76 prostate cancer patients and 71,500 nonlinear finite-element simulations from 143 different patients were used for this study. We show that, with only gland segmentation as training labels, the proposed method can help predict physically plausible deformation without any other smoothness penalty. Based on cross-validation experiments using 834 pairs of independent validation landmarks, the proposed adversarial-regularized registration achieved a target registration error of 6.3 mm that is significantly lower than those from several other regularization methods.
Ω-Net : Fully Automatic, Multi-View Cardiac MR Detection, Orientation, and Segmentation with Deep Neural Networks
Mar 20, 2018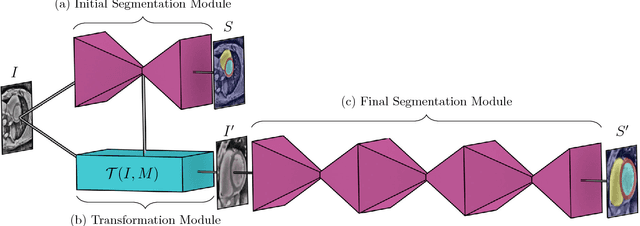
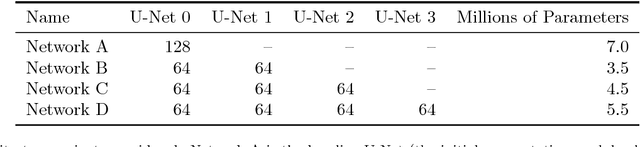
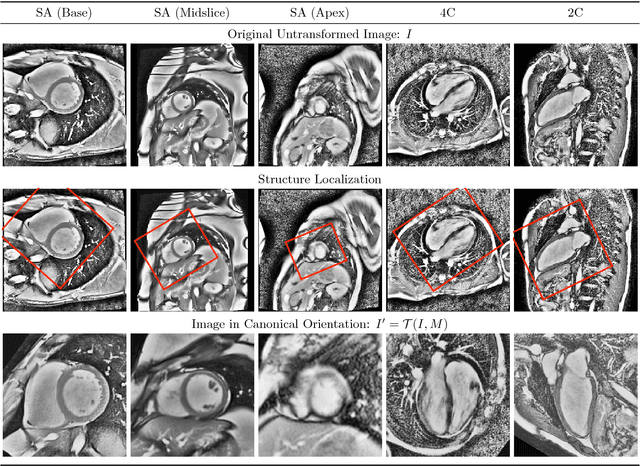
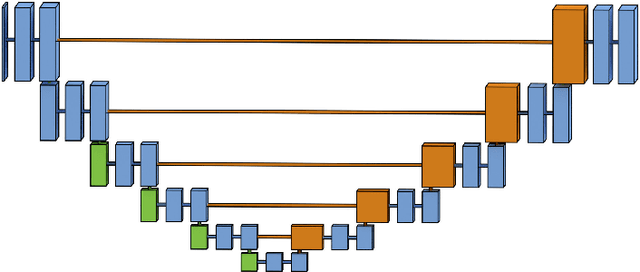
Abstract:Pixelwise segmentation of the left ventricular (LV) myocardium and the four cardiac chambers in 2-D steady state free precession (SSFP) cine sequences is an essential preprocessing step for a wide range of analyses. Variability in contrast, appearance, orientation, and placement of the heart between patients, clinical views, scanners, and protocols makes fully automatic semantic segmentation a notoriously difficult problem. Here, we present ${\Omega}$-Net (Omega-Net): a novel convolutional neural network (CNN) architecture for simultaneous localization, transformation into a canonical orientation, and semantic segmentation. First, an initial segmentation is performed on the input image, second, the features learned during this initial segmentation are used to predict the parameters needed to transform the input image into a canonical orientation, and third, a final segmentation is performed on the transformed image. In this work, ${\Omega}$-Nets of varying depths were trained to detect five foreground classes in any of three clinical views (short axis, SA, four-chamber, 4C, two-chamber, 2C), without prior knowledge of the view being segmented. The architecture was trained on a cohort of patients with hypertrophic cardiomyopathy and healthy control subjects. Network performance as measured by weighted foreground intersection-over-union (IoU) was substantially improved in the best-performing ${\Omega}$- Net compared with U-Net segmentation without localization or orientation. In addition, {\Omega}-Net was retrained from scratch on the 2017 MICCAI ACDC dataset, and achieves state-of-the-art results on the LV and RV bloodpools, and performed slightly worse in segmentation of the LV myocardium. We conclude this architecture represents a substantive advancement over prior approaches, with implications for biomedical image segmentation more generally.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge