cancer detection
Cancer detection using Artificial Intelligence (AI) involves leveraging advanced machine learning algorithms and techniques to identify and diagnose cancer from various medical data sources. The goal is to enhance early detection, improve diagnostic accuracy, and potentially reduce the need for invasive procedures.
Papers and Code
Dark-Field X-Ray Imaging Significantly Improves Deep-Learning based Detection of Synthetic Early-Stage Lung Tumors in Preclinical Models
Oct 31, 2025Low-dose computed tomography (LDCT) is the current standard for lung cancer screening, yet its adoption and accessibility remain limited. Many regions lack LDCT infrastructure, and even among those screened, early-stage cancer detection often yield false positives, as shown in the National Lung Screening Trial (NLST) with a sensitivity of 93.8 percent and a false-positive rate of 26.6 percent. We aim to investigate whether X-ray dark-field imaging (DFI) radiograph, a technique sensitive to small-angle scatter from alveolar microstructure and less susceptible to organ shadowing, can significantly improve early-stage lung tumor detection when coupled with deep-learning segmentation. Using paired attenuation (ATTN) and DFI radiograph images of euthanized mouse lungs, we generated realistic synthetic tumors with irregular boundaries and intensity profiles consistent with physical lung contrast. A U-Net segmentation network was trained on small patches using either ATTN, DFI, or a combination of ATTN and DFI channels. Results show that the DFI-only model achieved a true-positive detection rate of 83.7 percent, compared with 51 percent for ATTN-only, while maintaining comparable specificity (90.5 versus 92.9 percent). The combined ATTN and DFI input achieved 79.6 percent sensitivity and 97.6 percent specificity. In conclusion, DFI substantially improves early-tumor detectability in comparison to standard attenuation radiography and shows potential as an accessible, low-cost, low-dose alternative for pre-clinical or limited-resource screening where LDCT is unavailable.
Dynamic Weight Adjustment for Knowledge Distillation: Leveraging Vision Transformer for High-Accuracy Lung Cancer Detection and Real-Time Deployment
Oct 23, 2025This paper presents the FuzzyDistillViT-MobileNet model, a novel approach for lung cancer (LC) classification, leveraging dynamic fuzzy logic-driven knowledge distillation (KD) to address uncertainty and complexity in disease diagnosis. Unlike traditional models that rely on static KD with fixed weights, our method dynamically adjusts the distillation weight using fuzzy logic, enabling the student model to focus on high-confidence regions while reducing attention to ambiguous areas. This dynamic adjustment improves the model ability to handle varying uncertainty levels across different regions of LC images. We employ the Vision Transformer (ViT-B32) as the instructor model, which effectively transfers knowledge to the student model, MobileNet, enhancing the student generalization capabilities. The training process is further optimized using a dynamic wait adjustment mechanism that adapts the training procedure for improved convergence and performance. To enhance image quality, we introduce pixel-level image fusion improvement techniques such as Gamma correction and Histogram Equalization. The processed images (Pix1 and Pix2) are fused using a wavelet-based fusion method to improve image resolution and feature preservation. This fusion method uses the wavedec2 function to standardize images to a 224x224 resolution, decompose them into multi-scale frequency components, and recursively average coefficients at each level for better feature representation. To address computational efficiency, Genetic Algorithm (GA) is used to select the most suitable pre-trained student model from a pool of 12 candidates, balancing model performance with computational cost. The model is evaluated on two datasets, including LC25000 histopathological images (99.16% accuracy) and IQOTH/NCCD CT-scan images (99.54% accuracy), demonstrating robustness across different imaging domains.
MV-MLM: Bridging Multi-View Mammography and Language for Breast Cancer Diagnosis and Risk Prediction
Oct 30, 2025



Large annotated datasets are essential for training robust Computer-Aided Diagnosis (CAD) models for breast cancer detection or risk prediction. However, acquiring such datasets with fine-detailed annotation is both costly and time-consuming. Vision-Language Models (VLMs), such as CLIP, which are pre-trained on large image-text pairs, offer a promising solution by enhancing robustness and data efficiency in medical imaging tasks. This paper introduces a novel Multi-View Mammography and Language Model for breast cancer classification and risk prediction, trained on a dataset of paired mammogram images and synthetic radiology reports. Our MV-MLM leverages multi-view supervision to learn rich representations from extensive radiology data by employing cross-modal self-supervision across image-text pairs. This includes multiple views and the corresponding pseudo-radiology reports. We propose a novel joint visual-textual learning strategy to enhance generalization and accuracy performance over different data types and tasks to distinguish breast tissues or cancer characteristics(calcification, mass) and utilize these patterns to understand mammography images and predict cancer risk. We evaluated our method on both private and publicly available datasets, demonstrating that the proposed model achieves state-of-the-art performance in three classification tasks: (1) malignancy classification, (2) subtype classification, and (3) image-based cancer risk prediction. Furthermore, the model exhibits strong data efficiency, outperforming existing fully supervised or VLM baselines while trained on synthetic text reports and without the need for actual radiology reports.
RRTS Dataset: A Benchmark Colonoscopy Dataset from Resource-Limited Settings for Computer-Aided Diagnosis Research
Nov 10, 2025Background and Objective: Colorectal cancer prevention relies on early detection of polyps during colonoscopy. Existing public datasets, such as CVC-ClinicDB and Kvasir-SEG, provide valuable benchmarks but are limited by small sample sizes, curated image selection, or lack of real-world artifacts. There remains a need for datasets that capture the complexity of clinical practice, particularly in resource-constrained settings. Methods: We introduce a dataset, BUET Polyp Dataset (BPD), of colonoscopy images collected using Olympus 170 and Pentax i-Scan series endoscopes under routine clinical conditions. The dataset contains images with corresponding expert-annotated binary masks, reflecting diverse challenges such as motion blur, specular highlights, stool artifacts, blood, and low-light frames. Annotations were manually reviewed by clinical experts to ensure quality. To demonstrate baseline performance, we provide benchmark results for classification using VGG16, ResNet50, and InceptionV3, and for segmentation using UNet variants with VGG16, ResNet34, and InceptionV4 backbones. Results: The dataset comprises 1,288 images with polyps from 164 patients with corresponding ground-truth masks and 1,657 polyp-free images from 31 patients. Benchmarking experiments achieved up to 90.8% accuracy for binary classification (VGG16) and a maximum Dice score of 0.64 with InceptionV4-UNet for segmentation. Performance was lower compared to curated datasets, reflecting the real-world difficulty of images with artifacts and variable quality.
A Density-Informed Multimodal Artificial Intelligence Framework for Improving Breast Cancer Detection Across All Breast Densities
Oct 16, 2025Mammography, the current standard for breast cancer screening, has reduced sensitivity in women with dense breast tissue, contributing to missed or delayed diagnoses. Thermalytix, an AI-based thermal imaging modality, captures functional vascular and metabolic cues that may complement mammographic structural data. This study investigates whether a breast density-informed multi-modal AI framework can improve cancer detection by dynamically selecting the appropriate imaging modality based on breast tissue composition. A total of 324 women underwent both mammography and thermal imaging. Mammography images were analyzed using a multi-view deep learning model, while Thermalytix assessed thermal images through vascular and thermal radiomics. The proposed framework utilized Mammography AI for fatty breasts and Thermalytix AI for dense breasts, optimizing predictions based on tissue type. This multi-modal AI framework achieved a sensitivity of 94.55% (95% CI: 88.54-100) and specificity of 79.93% (95% CI: 75.14-84.71), outperforming standalone mammography AI (sensitivity 81.82%, specificity 86.25%) and Thermalytix AI (sensitivity 92.73%, specificity 75.46%). Importantly, the sensitivity of Mammography dropped significantly in dense breasts (67.86%) versus fatty breasts (96.30%), whereas Thermalytix AI maintained high and consistent sensitivity in both (92.59% and 92.86%, respectively). This demonstrates that a density-informed multi-modal AI framework can overcome key limitations of unimodal screening and deliver high performance across diverse breast compositions. The proposed framework is interpretable, low-cost, and easily deployable, offering a practical path to improving breast cancer screening outcomes in both high-resource and resource-limited settings.
H-CNN-ViT: A Hierarchical Gated Attention Multi-Branch Model for Bladder Cancer Recurrence Prediction
Nov 19, 2025Bladder cancer is one of the most prevalent malignancies worldwide, with a recurrence rate of up to 78%, necessitating accurate post-operative monitoring for effective patient management. Multi-sequence contrast-enhanced MRI is commonly used for recurrence detection; however, interpreting these scans remains challenging, even for experienced radiologists, due to post-surgical alterations such as scarring, swelling, and tissue remodeling. AI-assisted diagnostic tools have shown promise in improving bladder cancer recurrence prediction, yet progress in this field is hindered by the lack of dedicated multi-sequence MRI datasets for recurrence assessment study. In this work, we first introduce a curated multi-sequence, multi-modal MRI dataset specifically designed for bladder cancer recurrence prediction, establishing a valuable benchmark for future research. We then propose H-CNN-ViT, a new Hierarchical Gated Attention Multi-Branch model that enables selective weighting of features from the global (ViT) and local (CNN) paths based on contextual demands, achieving a balanced and targeted feature fusion. Our multi-branch architecture processes each modality independently, ensuring that the unique properties of each imaging channel are optimally captured and integrated. Evaluated on our dataset, H-CNN-ViT achieves an AUC of 78.6%, surpassing state-of-the-art models. Our model is publicly available at https://github.com/XLIAaron/H-CNN-ViT.
Histology-informed tiling of whole tissue sections improves the interpretability and predictability of cancer relapse and genetic alterations
Nov 13, 2025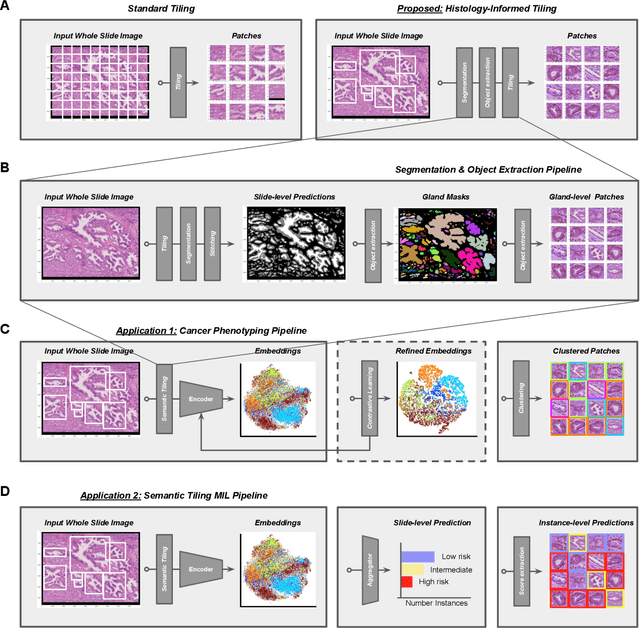
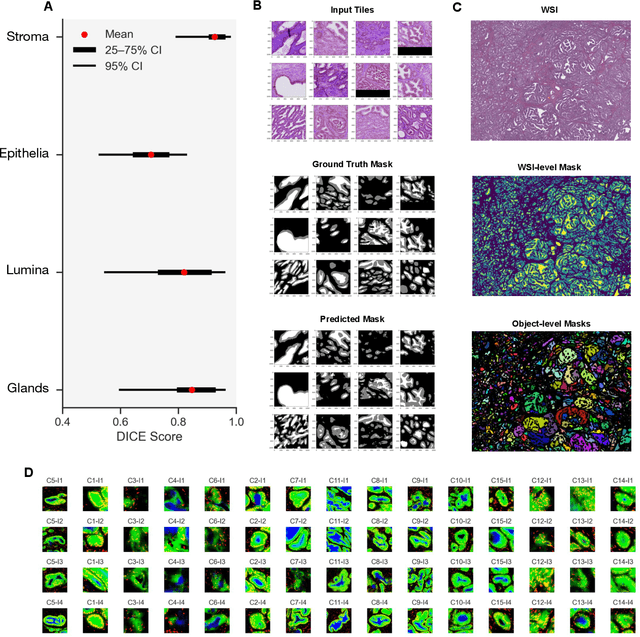
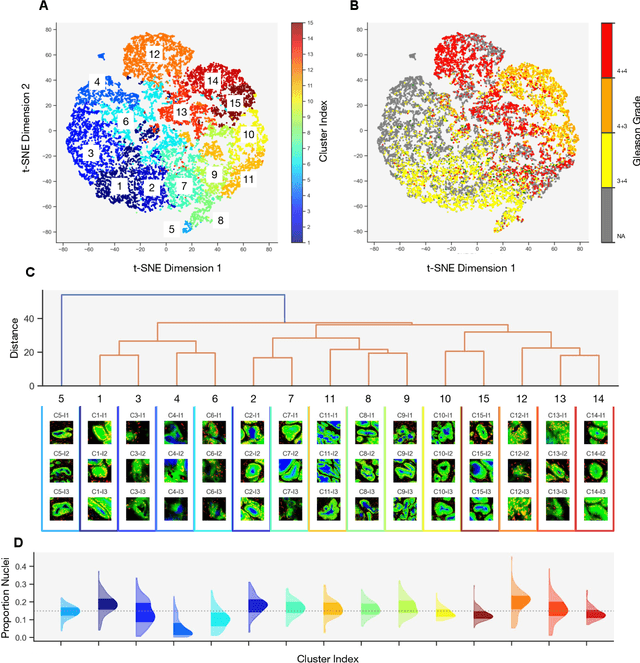
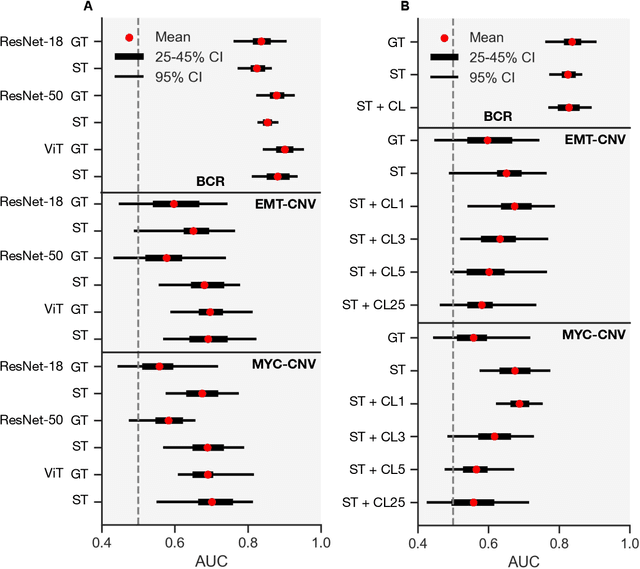
Histopathologists establish cancer grade by assessing histological structures, such as glands in prostate cancer. Yet, digital pathology pipelines often rely on grid-based tiling that ignores tissue architecture. This introduces irrelevant information and limits interpretability. We introduce histology-informed tiling (HIT), which uses semantic segmentation to extract glands from whole slide images (WSIs) as biologically meaningful input patches for multiple-instance learning (MIL) and phenotyping. Trained on 137 samples from the ProMPT cohort, HIT achieved a gland-level Dice score of 0.83 +/- 0.17. By extracting 380,000 glands from 760 WSIs across ICGC-C and TCGA-PRAD cohorts, HIT improved MIL models AUCs by 10% for detecting copy number variation (CNVs) in genes related to epithelial-mesenchymal transitions (EMT) and MYC, and revealed 15 gland clusters, several of which were associated with cancer relapse, oncogenic mutations, and high Gleason. Therefore, HIT improved the accuracy and interpretability of MIL predictions, while streamlining computations by focussing on biologically meaningful structures during feature extraction.
Artificial Intelligence-Enabled Analysis of Radiology Reports: Epidemiology and Consequences of Incidental Thyroid Findings
Oct 30, 2025



Importance Incidental thyroid findings (ITFs) are increasingly detected on imaging performed for non-thyroid indications. Their prevalence, features, and clinical consequences remain undefined. Objective To develop, validate, and deploy a natural language processing (NLP) pipeline to identify ITFs in radiology reports and assess their prevalence, features, and clinical outcomes. Design, Setting, and Participants Retrospective cohort of adults without prior thyroid disease undergoing thyroid-capturing imaging at Mayo Clinic sites from July 1, 2017, to September 30, 2023. A transformer-based NLP pipeline identified ITFs and extracted nodule characteristics from image reports from multiple modalities and body regions. Main Outcomes and Measures Prevalence of ITFs, downstream thyroid ultrasound, biopsy, thyroidectomy, and thyroid cancer diagnosis. Logistic regression identified demographic and imaging-related factors. Results Among 115,683 patients (mean age, 56.8 [SD 17.2] years; 52.9% women), 9,077 (7.8%) had an ITF, of which 92.9% were nodules. ITFs were more likely in women, older adults, those with higher BMI, and when imaging was ordered by oncology or internal medicine. Compared with chest CT, ITFs were more likely via neck CT, PET, and nuclear medicine scans. Nodule characteristics were poorly documented, with size reported in 44% and other features in fewer than 15% (e.g. calcifications). Compared with patients without ITFs, those with ITFs had higher odds of thyroid nodule diagnosis, biopsy, thyroidectomy and thyroid cancer diagnosis. Most cancers were papillary, and larger when detected after ITFs vs no ITF. Conclusions ITFs were common and strongly associated with cascades leading to the detection of small, low-risk cancers. These findings underscore the role of ITFs in thyroid cancer overdiagnosis and the need for standardized reporting and more selective follow-up.
Rank-Aware Agglomeration of Foundation Models for Immunohistochemistry Image Cell Counting
Nov 16, 2025Accurate cell counting in immunohistochemistry (IHC) images is critical for quantifying protein expression and aiding cancer diagnosis. However, the task remains challenging due to the chromogen overlap, variable biomarker staining, and diverse cellular morphologies. Regression-based counting methods offer advantages over detection-based ones in handling overlapped cells, yet rarely support end-to-end multi-class counting. Moreover, the potential of foundation models remains largely underexplored in this paradigm. To address these limitations, we propose a rank-aware agglomeration framework that selectively distills knowledge from multiple strong foundation models, leveraging their complementary representations to handle IHC heterogeneity and obtain a compact yet effective student model, CountIHC. Unlike prior task-agnostic agglomeration strategies that either treat all teachers equally or rely on feature similarity, we design a Rank-Aware Teacher Selecting (RATS) strategy that models global-to-local patch rankings to assess each teacher's inherent counting capacity and enable sample-wise teacher selection. For multi-class cell counting, we introduce a fine-tuning stage that reformulates the task as vision-language alignment. Discrete semantic anchors derived from structured text prompts encode both category and quantity information, guiding the regression of class-specific density maps and improving counting for overlapping cells. Extensive experiments demonstrate that CountIHC surpasses state-of-the-art methods across 12 IHC biomarkers and 5 tissue types, while exhibiting high agreement with pathologists' assessments. Its effectiveness on H&E-stained data further confirms the scalability of the proposed method.
Scale-Aware Curriculum Learning for Ddata-Efficient Lung Nodule Detection with YOLOv11
Oct 30, 2025Lung nodule detection in chest CT is crucial for early lung cancer diagnosis, yet existing deep learning approaches face challenges when deployed in clinical settings with limited annotated data. While curriculum learning has shown promise in improving model training, traditional static curriculum strategies fail in data-scarce scenarios. We propose Scale Adaptive Curriculum Learning (SACL), a novel training strategy that dynamically adjusts curriculum design based on available data scale. SACL introduces three key mechanisms:(1) adaptive epoch scheduling, (2) hard sample injection, and (3) scale-aware optimization. We evaluate SACL on the LUNA25 dataset using YOLOv11 as the base detector. Experimental results demonstrate that while SACL achieves comparable performance to static curriculum learning on the full dataset in mAP50, it shows significant advantages under data-limited conditions with 4.6%, 3.5%, and 2.0% improvements over baseline at 10%, 20%, and 50% of training data respectively. By enabling robust training across varying data scales without architectural modifications, SACL provides a practical solution for healthcare institutions to develop effective lung nodule detection systems despite limited annotation resources.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge