cancer detection
Cancer detection using Artificial Intelligence (AI) involves leveraging advanced machine learning algorithms and techniques to identify and diagnose cancer from various medical data sources. The goal is to enhance early detection, improve diagnostic accuracy, and potentially reduce the need for invasive procedures.
Papers and Code
AI-assisted Early Detection of Pancreatic Ductal Adenocarcinoma on Contrast-enhanced CT
Mar 13, 2025



Pancreatic ductal adenocarcinoma (PDAC) is one of the most common and aggressive types of pancreatic cancer. However, due to the lack of early and disease-specific symptoms, most patients with PDAC are diagnosed at an advanced disease stage. Consequently, early PDAC detection is crucial for improving patients' quality of life and expanding treatment options. In this work, we develop a coarse-to-fine approach to detect PDAC on contrast-enhanced CT scans. First, we localize and crop the region of interest from the low-resolution images, and then segment the PDAC-related structures at a finer scale. Additionally, we introduce two strategies to further boost detection performance: (1) a data-splitting strategy for model ensembling, and (2) a customized post-processing function. We participated in the PANORAMA challenge and ranked 1st place for PDAC detection with an AUROC of 0.9263 and an AP of 0.7243. Our code and models are publicly available at https://github.com/han-liu/PDAC_detection.
"No negatives needed": weakly-supervised regression for interpretable tumor detection in whole-slide histopathology images
Feb 28, 2025



Accurate tumor detection in digital pathology whole-slide images (WSIs) is crucial for cancer diagnosis and treatment planning. Multiple Instance Learning (MIL) has emerged as a widely used approach for weakly-supervised tumor detection with large-scale data without the need for manual annotations. However, traditional MIL methods often depend on classification tasks that require tumor-free cases as negative examples, which are challenging to obtain in real-world clinical workflows, especially for surgical resection specimens. We address this limitation by reformulating tumor detection as a regression task, estimating tumor percentages from WSIs, a clinically available target across multiple cancer types. In this paper, we provide an analysis of the proposed weakly-supervised regression framework by applying it to multiple organs, specimen types and clinical scenarios. We characterize the robustness of our framework to tumor percentage as a noisy regression target, and introduce a novel concept of amplification technique to improve tumor detection sensitivity when learning from small tumor regions. Finally, we provide interpretable insights into the model's predictions by analyzing visual attention and logit maps. Our code is available at https://github.com/DIAGNijmegen/tumor-percentage-mil-regression.
Robust Polyp Detection and Diagnosis through Compositional Prompt-Guided Diffusion Models
Feb 25, 2025Colorectal cancer (CRC) is a significant global health concern, and early detection through screening plays a critical role in reducing mortality. While deep learning models have shown promise in improving polyp detection, classification, and segmentation, their generalization across diverse clinical environments, particularly with out-of-distribution (OOD) data, remains a challenge. Multi-center datasets like PolypGen have been developed to address these issues, but their collection is costly and time-consuming. Traditional data augmentation techniques provide limited variability, failing to capture the complexity of medical images. Diffusion models have emerged as a promising solution for generating synthetic polyp images, but the image generation process in current models mainly relies on segmentation masks as the condition, limiting their ability to capture the full clinical context. To overcome these limitations, we propose a Progressive Spectrum Diffusion Model (PSDM) that integrates diverse clinical annotations-such as segmentation masks, bounding boxes, and colonoscopy reports-by transforming them into compositional prompts. These prompts are organized into coarse and fine components, allowing the model to capture both broad spatial structures and fine details, generating clinically accurate synthetic images. By augmenting training data with PSDM-generated samples, our model significantly improves polyp detection, classification, and segmentation. For instance, on the PolypGen dataset, PSDM increases the F1 score by 2.12% and the mean average precision by 3.09%, demonstrating superior performance in OOD scenarios and enhanced generalization.
Graph Kolmogorov-Arnold Networks for Multi-Cancer Classification and Biomarker Identification, An Interpretable Multi-Omics Approach
Mar 29, 2025The integration of multi-omics data presents a major challenge in precision medicine, requiring advanced computational methods for accurate disease classification and biological interpretation. This study introduces the Multi-Omics Graph Kolmogorov-Arnold Network (MOGKAN), a deep learning model that integrates messenger RNA, micro RNA sequences, and DNA methylation data with Protein-Protein Interaction (PPI) networks for accurate and interpretable cancer classification across 31 cancer types. MOGKAN employs a hybrid approach combining differential expression with DESeq2, Linear Models for Microarray (LIMMA), and Least Absolute Shrinkage and Selection Operator (LASSO) regression to reduce multi-omics data dimensionality while preserving relevant biological features. The model architecture is based on the Kolmogorov-Arnold theorem principle, using trainable univariate functions to enhance interpretability and feature analysis. MOGKAN achieves classification accuracy of 96.28 percent and demonstrates low experimental variability with a standard deviation that is reduced by 1.58 to 7.30 percents compared to Convolutional Neural Networks (CNNs) and Graph Neural Networks (GNNs). The biomarkers identified by MOGKAN have been validated as cancer-related markers through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The proposed model presents an ability to uncover molecular oncogenesis mechanisms by detecting phosphoinositide-binding substances and regulating sphingolipid cellular processes. By integrating multi-omics data with graph-based deep learning, our proposed approach demonstrates superior predictive performance and interpretability that has the potential to enhance the translation of complex multi-omics data into clinically actionable cancer diagnostics.
Style transfer as data augmentation: evaluating unpaired image-to-image translation models in mammography
Feb 04, 2025



Several studies indicate that deep learning models can learn to detect breast cancer from mammograms (X-ray images of the breasts). However, challenges with overfitting and poor generalisability prevent their routine use in the clinic. Models trained on data from one patient population may not perform well on another due to differences in their data domains, emerging due to variations in scanning technology or patient characteristics. Data augmentation techniques can be used to improve generalisability by expanding the diversity of feature representations in the training data by altering existing examples. Image-to-image translation models are one approach capable of imposing the characteristic feature representations (i.e. style) of images from one dataset onto another. However, evaluating model performance is non-trivial, particularly in the absence of ground truths (a common reality in medical imaging). Here, we describe some key aspects that should be considered when evaluating style transfer algorithms, highlighting the advantages and disadvantages of popular metrics, and important factors to be mindful of when implementing them in practice. We consider two types of generative models: a cycle-consistent generative adversarial network (CycleGAN) and a diffusion-based SynDiff model. We learn unpaired image-to-image translation across three mammography datasets. We highlight that undesirable aspects of model performance may determine the suitability of some metrics, and also provide some analysis indicating the extent to which various metrics assess unique aspects of model performance. We emphasise the need to use several metrics for a comprehensive assessment of model performance.
From Slices to Sequences: Autoregressive Tracking Transformer for Cohesive and Consistent 3D Lymph Node Detection in CT Scans
Mar 11, 2025



Lymph node (LN) assessment is an essential task in the routine radiology workflow, providing valuable insights for cancer staging, treatment planning and beyond. Identifying scatteredly-distributed and low-contrast LNs in 3D CT scans is highly challenging, even for experienced clinicians. Previous lesion and LN detection methods demonstrate effectiveness of 2.5D approaches (i.e, using 2D network with multi-slice inputs), leveraging pretrained 2D model weights and showing improved accuracy as compared to separate 2D or 3D detectors. However, slice-based 2.5D detectors do not explicitly model inter-slice consistency for LN as a 3D object, requiring heuristic post-merging steps to generate final 3D LN instances, which can involve tuning a set of parameters for each dataset. In this work, we formulate 3D LN detection as a tracking task and propose LN-Tracker, a novel LN tracking transformer, for joint end-to-end detection and 3D instance association. Built upon DETR-based detector, LN-Tracker decouples transformer decoder's query into the track and detection groups, where the track query autoregressively follows previously tracked LN instances along the z-axis of a CT scan. We design a new transformer decoder with masked attention module to align track query's content to the context of current slice, meanwhile preserving detection query's high accuracy in current slice. An inter-slice similarity loss is introduced to encourage cohesive LN association between slices. Extensive evaluation on four lymph node datasets shows LN-Tracker's superior performance, with at least 2.7% gain in average sensitivity when compared to other top 3D/2.5D detectors. Further validation on public lung nodule and prostate tumor detection tasks confirms the generalizability of LN-Tracker as it achieves top performance on both tasks. Datasets will be released upon acceptance.
Towards Fair Medical AI: Adversarial Debiasing of 3D CT Foundation Embeddings
Feb 05, 2025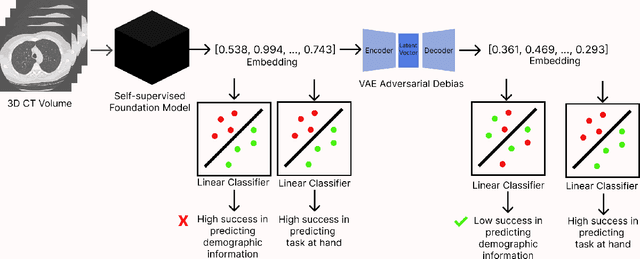
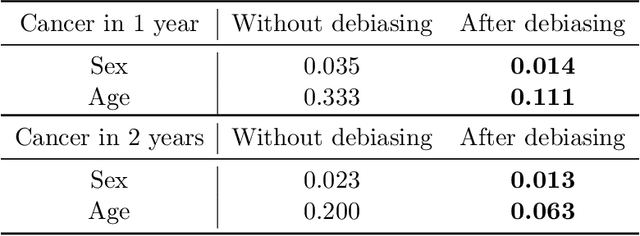
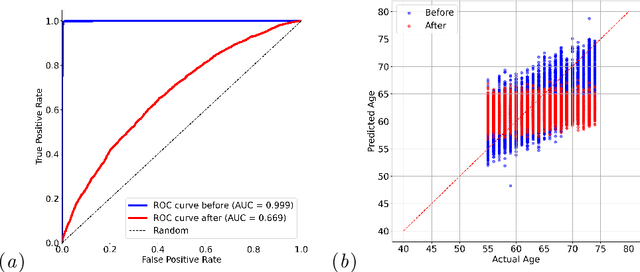
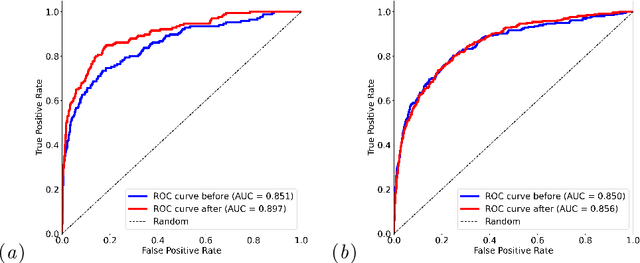
Self-supervised learning has revolutionized medical imaging by enabling efficient and generalizable feature extraction from large-scale unlabeled datasets. Recently, self-supervised foundation models have been extended to three-dimensional (3D) computed tomography (CT) data, generating compact, information-rich embeddings with 1408 features that achieve state-of-the-art performance on downstream tasks such as intracranial hemorrhage detection and lung cancer risk forecasting. However, these embeddings have been shown to encode demographic information, such as age, sex, and race, which poses a significant risk to the fairness of clinical applications. In this work, we propose a Variation Autoencoder (VAE) based adversarial debiasing framework to transform these embeddings into a new latent space where demographic information is no longer encoded, while maintaining the performance of critical downstream tasks. We validated our approach on the NLST lung cancer screening dataset, demonstrating that the debiased embeddings effectively eliminate multiple encoded demographic information and improve fairness without compromising predictive accuracy for lung cancer risk at 1-year and 2-year intervals. Additionally, our approach ensures the embeddings are robust against adversarial bias attacks. These results highlight the potential of adversarial debiasing techniques to ensure fairness and equity in clinical applications of self-supervised 3D CT embeddings, paving the way for their broader adoption in unbiased medical decision-making.
Single Shot AI-assisted quantification of KI-67 proliferation index in breast cancer
Mar 25, 2025Reliable quantification of Ki-67, a key proliferation marker in breast cancer, is essential for molecular subtyping and informed treatment planning. Conventional approaches, including visual estimation and manual counting, suffer from interobserver variability and limited reproducibility. This study introduces an AI-assisted method using the YOLOv8 object detection framework for automated Ki-67 scoring. High-resolution digital images (40x magnification) of immunohistochemically stained tumor sections were captured from Ki-67 hotspot regions and manually annotated by a domain expert to distinguish Ki-67-positive and negative tumor cells. The dataset was augmented and divided into training (80%), validation (10%), and testing (10%) subsets. Among the YOLOv8 variants tested, the Medium model achieved the highest performance, with a mean Average Precision at 50% Intersection over Union (mAP50) exceeding 85% for Ki-67-positive cells. The proposed approach offers an efficient, scalable, and objective alternative to conventional scoring methods, supporting greater consistency in Ki-67 evaluation. Future directions include developing user-friendly clinical interfaces and expanding to multi-institutional datasets to enhance generalizability and facilitate broader adoption in diagnostic practice.
Tumor monitoring and detection of lymph node metastasis using quantitative ultrasound and immune cytokine profiling in dogs undergoing radiation therapy: a pilot study
Mar 25, 2025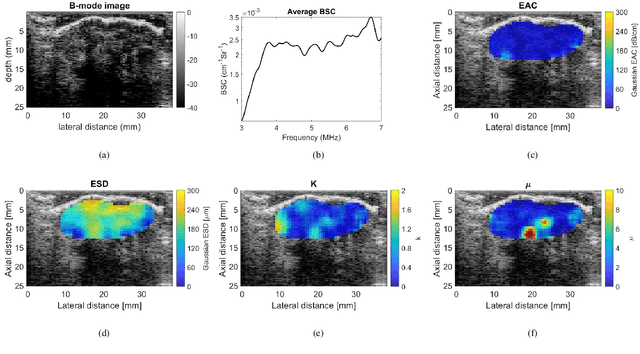
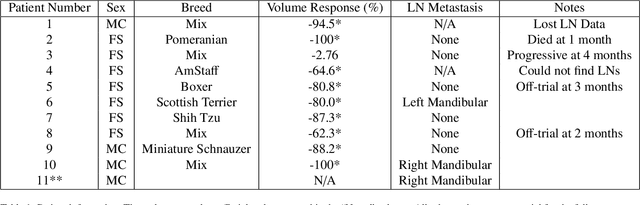

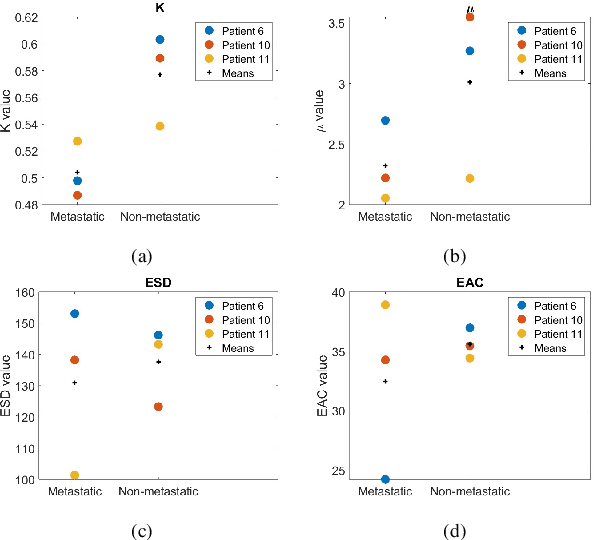
Quantitative ultrasound (QUS) characterizes the composition of cells to distinguish diseased from healthy tissue. QUS can reflect the complexity of the tumor and detect early lymph node (LN) metastasis ex vivo. The objective in this study was to gather preliminary QUS and cytokine data from dogs undergoing radiation therapy and correlate QUS data with both LN metastasis and tumor response. Spontaneous solid tumors were evaluated with QUS before and up to one year after receiving RT. Additionally, regional LNs were evaluated with QUS in vivo, then excised and examined with histopathology to detect metastasis. Paired t-tests were used to compare QUS data of metastatic and non-metastatic LNs within patients. Furthermore, paired t-tests compared pre- versus post-RT QUS data. Serum was collected at each time point for cytokine profiles. Most statistical tests were underpowered to produce significant p values, but interesting trends were observed. The lowest p values for LN tests were found with the envelope statistics K (p = 0.142) and mu (p = 0.181), which correspond to cell structure and number of scatterers. For tumor response, the lowest p values were found with K (p = 0.115) and mu (p = 0.127) when comparing baseline QUS data with QUS data 1 week after RT. Monocyte chemoattractant protein 1 (MCP-1) was significantly higher in dogs with cancer when compared to healthy controls (p = 1.12e-4). A weak correlation was found between effective scatterer diameter (ESD) and Transforming growth factor beta 1 (TGFB-1). While statistical tests on the preliminary QUS data alone were underpowered to detect significant differences among groups, our methods create a basis for future studies.
SMILE: a Scale-aware Multiple Instance Learning Method for Multicenter STAS Lung Cancer Histopathology Diagnosis
Mar 18, 2025Spread through air spaces (STAS) represents a newly identified aggressive pattern in lung cancer, which is known to be associated with adverse prognostic factors and complex pathological features. Pathologists currently rely on time consuming manual assessments, which are highly subjective and prone to variation. This highlights the urgent need for automated and precise diag nostic solutions. 2,970 lung cancer tissue slides are comprised from multiple centers, re-diagnosed them, and constructed and publicly released three lung cancer STAS datasets: STAS CSU (hospital), STAS TCGA, and STAS CPTAC. All STAS datasets provide corresponding pathological feature diagnoses and related clinical data. To address the bias, sparse and heterogeneous nature of STAS, we propose an scale-aware multiple instance learning(SMILE) method for STAS diagnosis of lung cancer. By introducing a scale-adaptive attention mechanism, the SMILE can adaptively adjust high attention instances, reducing over-reliance on local regions and promoting consistent detection of STAS lesions. Extensive experiments show that SMILE achieved competitive diagnostic results on STAS CSU, diagnosing 251 and 319 STAS samples in CPTAC andTCGA,respectively, surpassing clinical average AUC. The 11 open baseline results are the first to be established for STAS research, laying the foundation for the future expansion, interpretability, and clinical integration of computational pathology technologies. The datasets and code are available at https://anonymous.4open.science/r/IJCAI25-1DA1.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge