Xiaohua Chen
Research on Dead Reckoning Algorithm for Self-Propelled Pipeline Robots in Three-Dimensional Complex Pipelines
Dec 19, 2025



Abstract:In the field of gas pipeline location, existing pipeline location methods mostly rely on pipeline location instruments. However, when faced with complex and curved pipeline scenarios, these methods often fail due to problems such as cable entanglement and insufficient equipment flexibility. To address this pain point, we designed a self-propelled pipeline robot. This robot can autonomously complete the location work of complex and curved pipelines in complex pipe networks without external dragging. In terms of pipeline mapping technology, traditional visual mapping and laser mapping methods are easily affected by lighting conditions and insufficient features in the confined space of pipelines, resulting in mapping drift and divergence problems. In contrast, the pipeline location method that integrates inertial navigation and wheel odometers is less affected by pipeline environmental factors. Based on this, this paper proposes a pipeline robot location method based on extended Kalman filtering (EKF). Firstly, the body attitude angle is initially obtained through an inertial measurement unit (IMU). Then, the extended Kalman filtering algorithm is used to improve the accuracy of attitude angle estimation. Finally, high-precision pipeline location is achieved by combining wheel odometers. During the testing phase, the roll wheels of the pipeline robot needed to fit tightly against the pipe wall to reduce slippage. However, excessive tightness would reduce the flexibility of motion control due to excessive friction. Therefore, a balance needed to be struck between the robot's motion capability and positioning accuracy. Experiments were conducted using the self-propelled pipeline robot in a rectangular loop pipeline, and the results verified the effectiveness of the proposed dead reckoning algorithm.
Imagine by Reasoning: A Reasoning-Based Implicit Semantic Data Augmentation for Long-Tailed Classification
Dec 15, 2021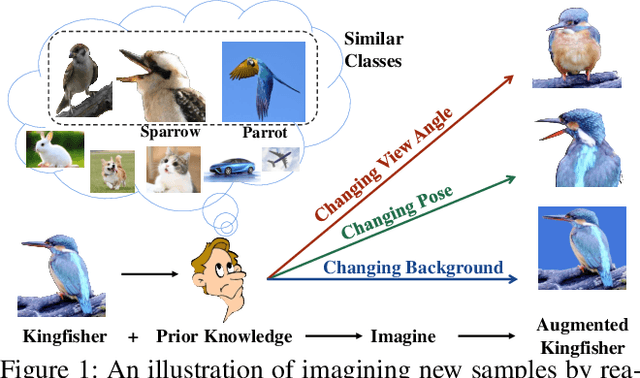
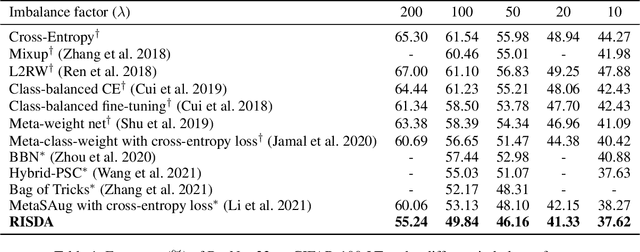
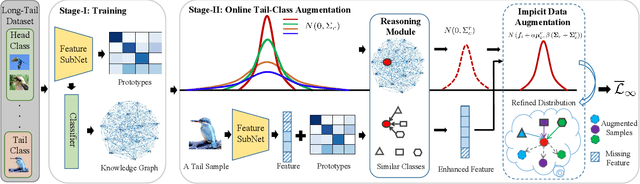
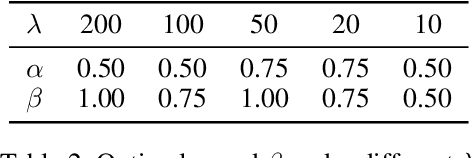
Abstract:Real-world data often follows a long-tailed distribution, which makes the performance of existing classification algorithms degrade heavily. A key issue is that samples in tail categories fail to depict their intra-class diversity. Humans can imagine a sample in new poses, scenes, and view angles with their prior knowledge even if it is the first time to see this category. Inspired by this, we propose a novel reasoning-based implicit semantic data augmentation method to borrow transformation directions from other classes. Since the covariance matrix of each category represents the feature transformation directions, we can sample new directions from similar categories to generate definitely different instances. Specifically, the long-tailed distributed data is first adopted to train a backbone and a classifier. Then, a covariance matrix for each category is estimated, and a knowledge graph is constructed to store the relations of any two categories. Finally, tail samples are adaptively enhanced via propagating information from all the similar categories in the knowledge graph. Experimental results on CIFAR-100-LT, ImageNet-LT, and iNaturalist 2018 have demonstrated the effectiveness of our proposed method compared with the state-of-the-art methods.
Comprehensive and Clinically Accurate Head and Neck Organs at Risk Delineation via Stratified Deep Learning: A Large-scale Multi-Institutional Study
Nov 01, 2021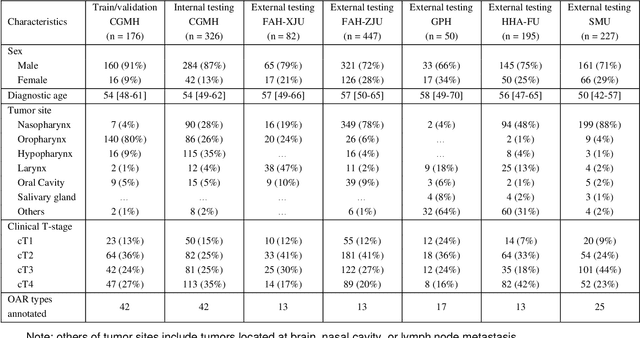
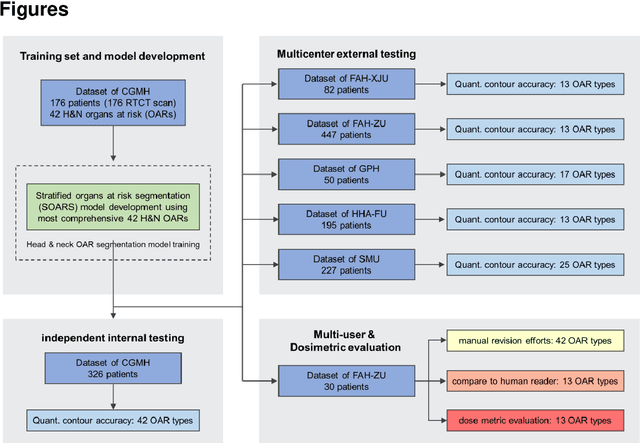
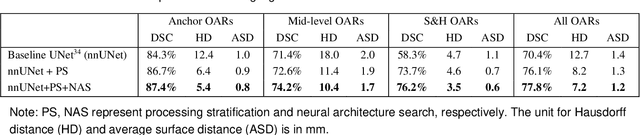
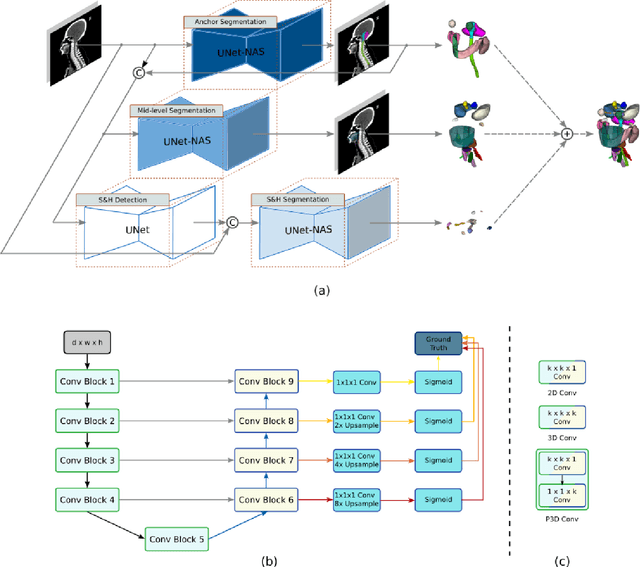
Abstract:Accurate organ at risk (OAR) segmentation is critical to reduce the radiotherapy post-treatment complications. Consensus guidelines recommend a set of more than 40 OARs in the head and neck (H&N) region, however, due to the predictable prohibitive labor-cost of this task, most institutions choose a substantially simplified protocol by delineating a smaller subset of OARs and neglecting the dose distributions associated with other OARs. In this work we propose a novel, automated and highly effective stratified OAR segmentation (SOARS) system using deep learning to precisely delineate a comprehensive set of 42 H&N OARs. SOARS stratifies 42 OARs into anchor, mid-level, and small & hard subcategories, with specifically derived neural network architectures for each category by neural architecture search (NAS) principles. We built SOARS models using 176 training patients in an internal institution and independently evaluated on 1327 external patients across six different institutions. It consistently outperformed other state-of-the-art methods by at least 3-5% in Dice score for each institutional evaluation (up to 36% relative error reduction in other metrics). More importantly, extensive multi-user studies evidently demonstrated that 98% of the SOARS predictions need only very minor or no revisions for direct clinical acceptance (saving 90% radiation oncologists workload), and their segmentation and dosimetric accuracy are within or smaller than the inter-user variation. These findings confirmed the strong clinical applicability of SOARS for the OAR delineation process in H&N cancer radiotherapy workflows, with improved efficiency, comprehensiveness, and quality.
Multi-institutional Validation of Two-Streamed Deep Learning Method for Automated Delineation of Esophageal Gross Tumor Volume using planning-CT and FDG-PETCT
Oct 11, 2021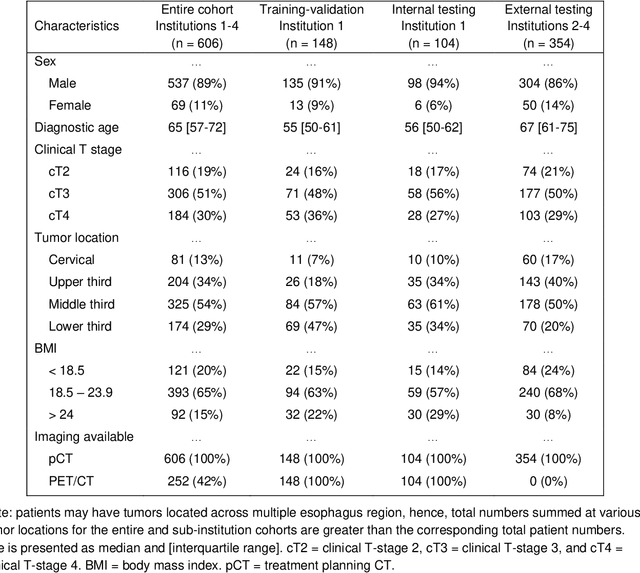
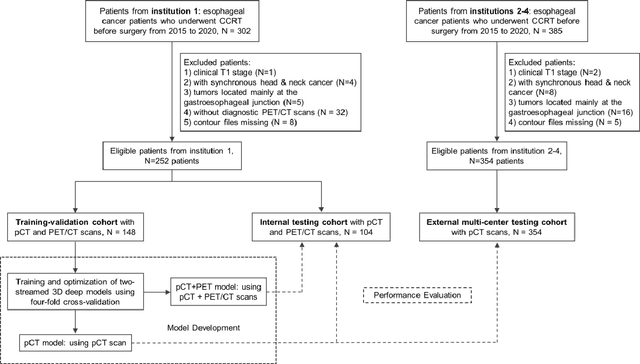
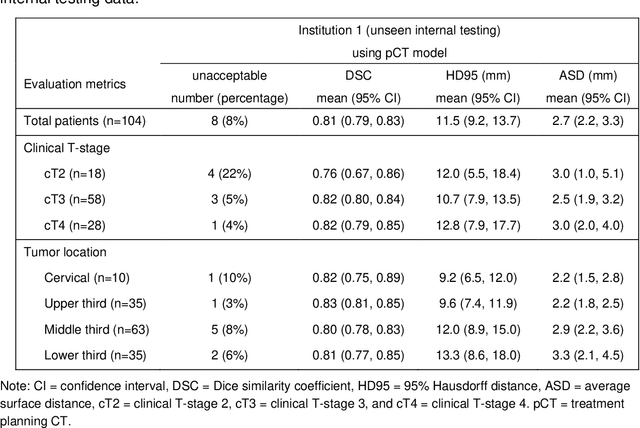
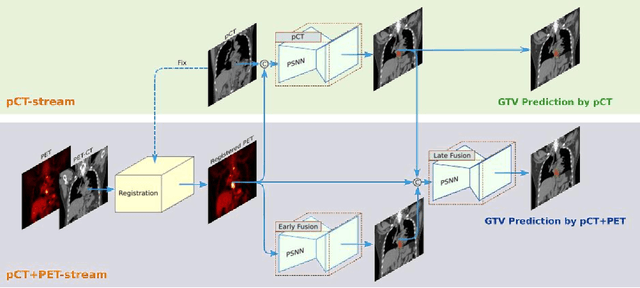
Abstract:Background: The current clinical workflow for esophageal gross tumor volume (GTV) contouring relies on manual delineation of high labor-costs and interuser variability. Purpose: To validate the clinical applicability of a deep learning (DL) multi-modality esophageal GTV contouring model, developed at 1 institution whereas tested at multiple ones. Methods and Materials: We collected 606 esophageal cancer patients from four institutions. 252 institution-1 patients had a treatment planning-CT (pCT) and a pair of diagnostic FDG-PETCT; 354 patients from other 3 institutions had only pCT. A two-streamed DL model for GTV segmentation was developed using pCT and PETCT scans of a 148 patient institution-1 subset. This built model had the flexibility of segmenting GTVs via only pCT or pCT+PETCT combined. For independent evaluation, the rest 104 institution-1 patients behaved as unseen internal testing, and 354 institutions 2-4 patients were used for external testing. We evaluated manual revision degrees by human experts to assess the contour-editing effort. The performance of the deep model was compared against 4 radiation oncologists in a multiuser study with 20 random external patients. Contouring accuracy and time were recorded for the pre-and post-DL assisted delineation process. Results: Our model achieved high segmentation accuracy in internal testing (mean Dice score: 0.81 using pCT and 0.83 using pCT+PET) and generalized well to external evaluation (mean DSC: 0.80). Expert assessment showed that the predicted contours of 88% patients need only minor or no revision. In multi-user evaluation, with the assistance of a deep model, inter-observer variation and required contouring time were reduced by 37.6% and 48.0%, respectively. Conclusions: Deep learning predicted GTV contours were in close agreement with the ground truth and could be adopted clinically with mostly minor or no changes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge