Wiro J. Niessen
Department of Radiology and Nuclear Medicine, Erasmus MC Cancer Institute, University Medical Center Rotterdam, Rotterdam, the Netherlands, Faculty of Medical Sciences, University of Groningen, Groningen, the Netherlands
WHO 2016 subtyping and automated segmentation of glioma using multi-task deep learning
Oct 09, 2020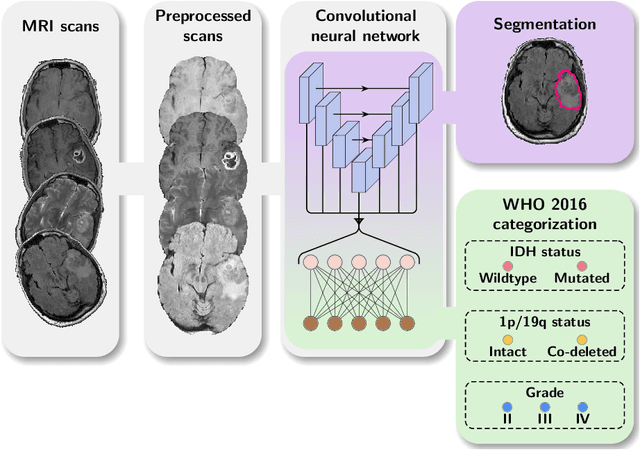
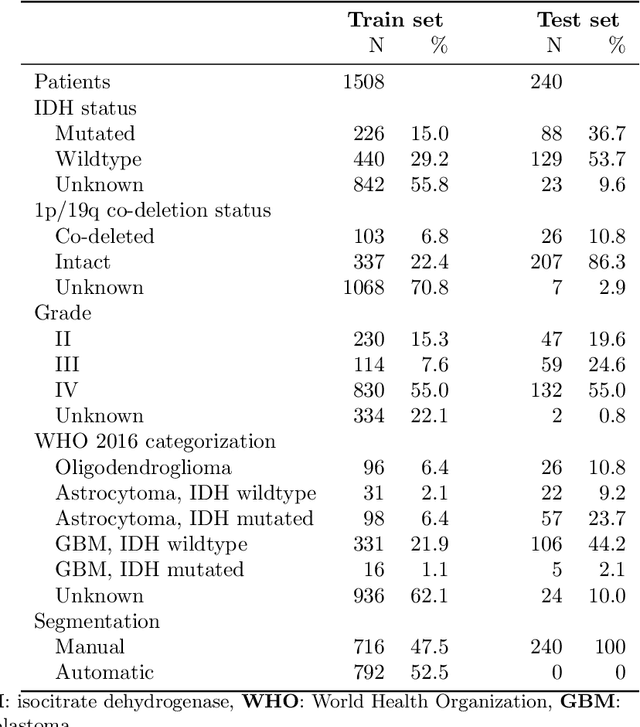
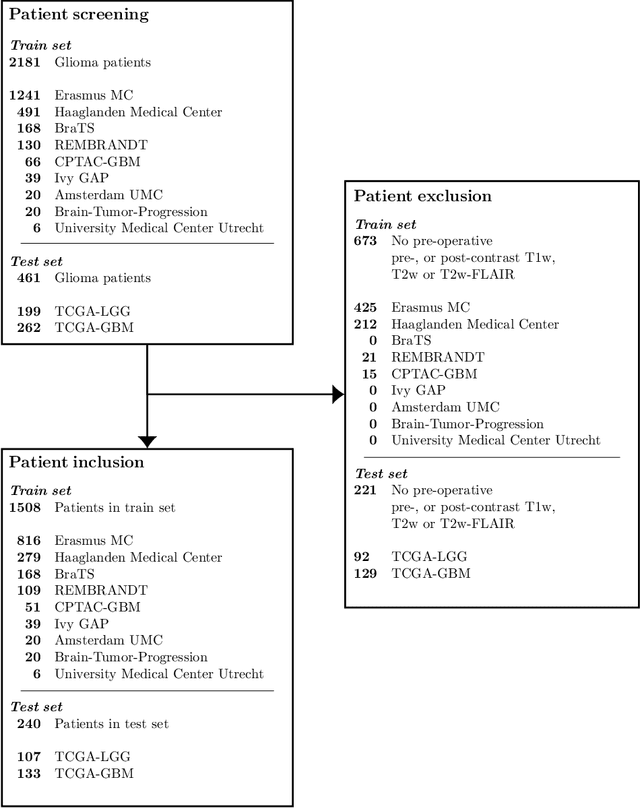
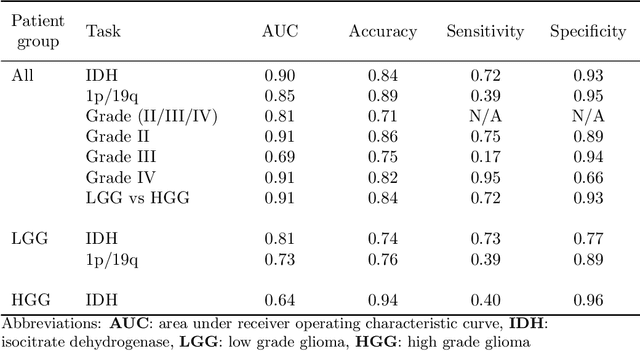
Abstract:Accurate characterization of glioma is crucial for clinical decision making. A delineation of the tumor is also desirable in the initial decision stages but is a time-consuming task. Leveraging the latest GPU capabilities, we developed a single multi-task convolutional neural network that uses the full 3D, structural, pre-operative MRI scans to can predict the IDH mutation status, the 1p/19q co-deletion status, and the grade of a tumor, while simultaneously segmenting the tumor. We trained our method using the largest, most diverse patient cohort to date containing 1508 glioma patients from 16 institutes. We tested our method on an independent dataset of 240 patients from 13 different institutes, and achieved an IDH-AUC of 0.90, 1p/19q-AUC of 0.85, grade-AUC of 0.81, and a mean whole tumor DICE score of 0.84. Thus, our method non-invasively predicts multiple, clinically relevant parameters and generalizes well to the broader clinical population.
autoTICI: Automatic Brain Tissue Reperfusion Scoring on 2D DSA Images of Acute Ischemic Stroke Patients
Oct 06, 2020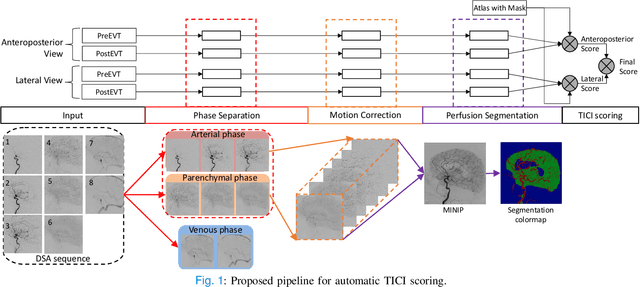
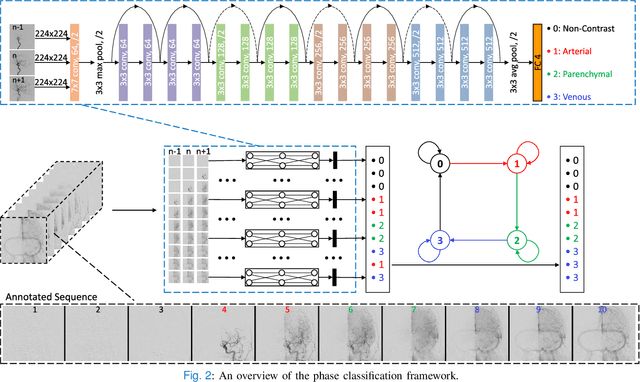
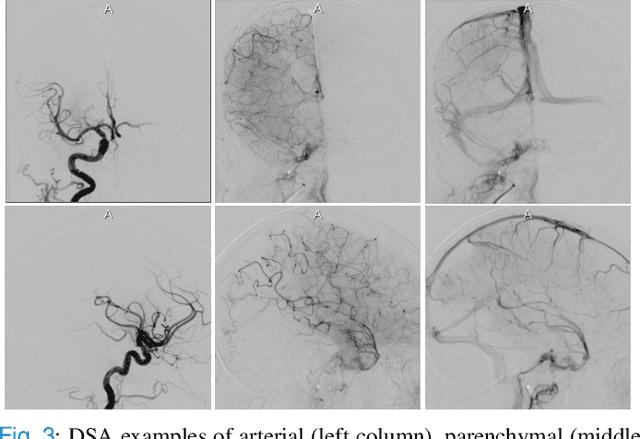
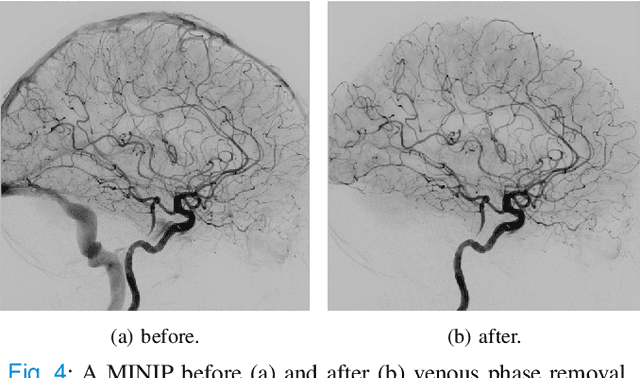
Abstract:The Thrombolysis in Cerebral Infarction (TICI) score is an important metric for reperfusion therapy assessment in acute ischemic stroke. It is commonly used as a technical outcome measure after endovascular treatment (EVT). Existing TICI scores are defined in coarse ordinal grades based on visual inspection, leading to inter- and intra-observer variation. In this work, we present autoTICI, an automatic and quantitative TICI scoring method. First, each digital subtraction angiography (DSA) sequence is separated into four phases (non-contrast, arterial, parenchymal and venous phase) using a multi-path convolutional neural network (CNN), which exploits spatio-temporal features. The network also incorporates sequence level label dependencies in the form of a state-transition matrix. Next, a minimum intensity map (MINIP) is computed using the motion corrected arterial and parenchymal frames. On the MINIP image, vessel, perfusion and background pixels are segmented. Finally, we quantify the autoTICI score as the ratio of reperfused pixels after EVT. On a routinely acquired multi-center dataset, the proposed autoTICI shows good correlation with the extended TICI (eTICI) reference with an average area under the curve (AUC) score of 0.81. The AUC score is 0.90 with respect to the dichotomized eTICI. In terms of clinical outcome prediction, we demonstrate that autoTICI is overall comparable to eTICI.
Analyzing the effect of APOE on Alzheimer's disease progression using an event-based model for stratified populations
Sep 15, 2020
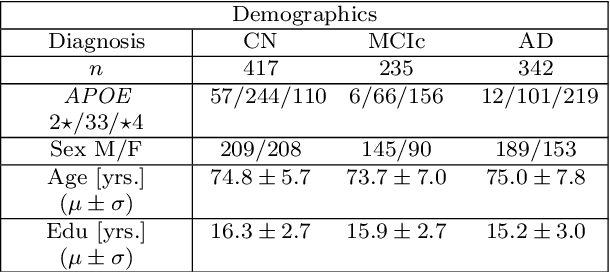
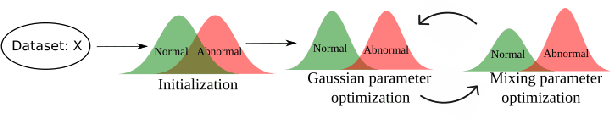
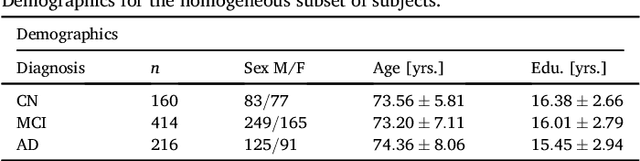
Abstract:Alzheimer's disease (AD) is the most common form of dementia and is phenotypically heterogeneous. APOE is a triallelic gene which correlates with phenotypic heterogeneity in AD. In this work, we determined the effect of APOE alleles on the disease progression timeline of AD using a discriminative event-based model (DEBM). Since DEBM is a data-driven model, stratification into smaller disease subgroups would lead to more inaccurate models as compared to fitting the model on the entire dataset. Hence our secondary aim is to propose and evaluate novel approaches in which we split the different steps of DEBM into group-aspecific and group-specific parts, where the entire dataset is used to train the group-aspecific parts and only the data from a specific group is used to train the group-specific parts of the DEBM. We performed simulation experiments to benchmark the accuracy of the proposed approaches and to select the optimal approach. Subsequently, the chosen approach was applied to the baseline data of 417 cognitively normal, 235 mild cognitively impaired who convert to AD within 3 years, and 342 AD patients from the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset to gain new insights into the effect of APOE carriership on the disease progression timeline of AD. The presented models could aid understanding of the disease, and in selecting homogeneous group of presymptomatic subjects at-risk of developing symptoms for clinical trials.
Neuro4Neuro: A neural network approach for neural tract segmentation using large-scale population-based diffusion imaging
May 26, 2020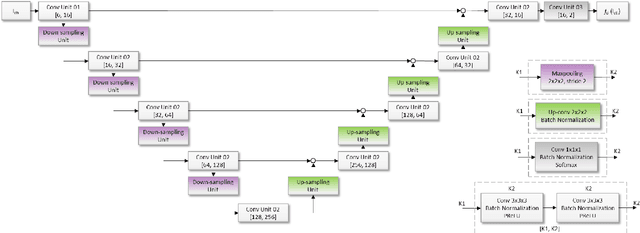
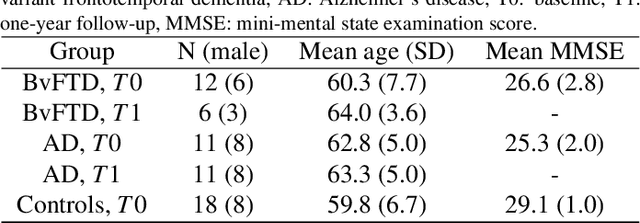
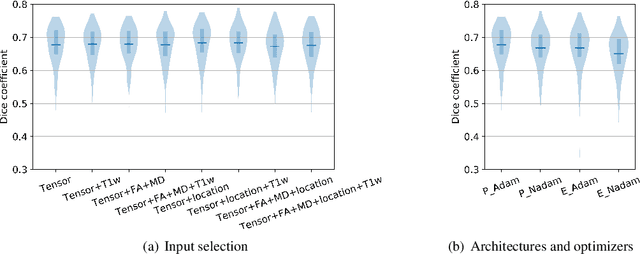
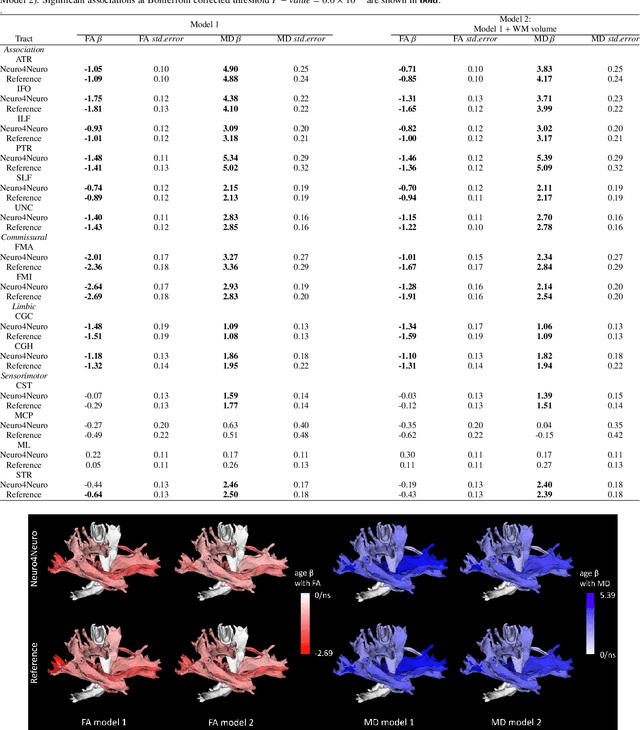
Abstract:Subtle changes in white matter (WM) microstructure have been associated with normal aging and neurodegeneration. To study these associations in more detail, it is highly important that the WM tracts can be accurately and reproducibly characterized from brain diffusion MRI. In addition, to enable analysis of WM tracts in large datasets and in clinical practice it is essential to have methodology that is fast and easy to apply. This work therefore presents a new approach for WM tract segmentation: Neuro4Neuro, that is capable of direct extraction of WM tracts from diffusion tensor images using convolutional neural network (CNN). This 3D end-to-end method is trained to segment 25 WM tracts in aging individuals from a large population-based study (N=9752, 1.5T MRI). The proposed method showed good segmentation performance and high reproducibility, i.e., a high spatial agreement (Cohen's kappa, k = 0.72 ~ 0.83) and a low scan-rescan error in tract-specific diffusion measures (e.g., fractional anisotropy: error = 1% ~ 5%). The reproducibility of the proposed method was higher than that of a tractography-based segmentation algorithm, while being orders of magnitude faster (0.5s to segment one tract). In addition, we showed that the method successfully generalizes to diffusion scans from an external dementia dataset (N=58, 3T MRI). In two proof-of-principle experiments, we associated WM microstructure obtained using the proposed method with age in a normal elderly population, and with disease subtypes in a dementia cohort. In concordance with the literature, results showed a widespread reduction of microstructural organization with aging and substantial group-wise microstructure differences between dementia subtypes. In conclusion, we presented a highly reproducible and fast method for WM tract segmentation that has the potential of being used in large-scale studies and clinical practice.
Towards segmentation and spatial alignment of the human embryonic brain using deep learning for atlas-based registration
May 13, 2020



Abstract:We propose an unsupervised deep learning method for atlas based registration to achieve segmentation and spatial alignment of the embryonic brain in a single framework. Our approach consists of two sequential networks with a specifically designed loss function to address the challenges in 3D first trimester ultrasound. The first part learns the affine transformation and the second part learns the voxelwise nonrigid deformation between the target image and the atlas. We trained this network end-to-end and validated it against a ground truth on synthetic datasets designed to resemble the challenges present in 3D first trimester ultrasound. The method was tested on a dataset of human embryonic ultrasound volumes acquired at 9 weeks gestational age, which showed alignment of the brain in some cases and gave insight in open challenges for the proposed method. We conclude that our method is a promising approach towards fully automated spatial alignment and segmentation of embryonic brains in 3D ultrasound.
When Weak Becomes Strong: Robust Quantification of White Matter Hyperintensities in Brain MRI scans
Apr 12, 2020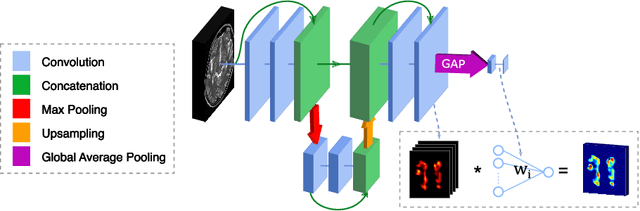
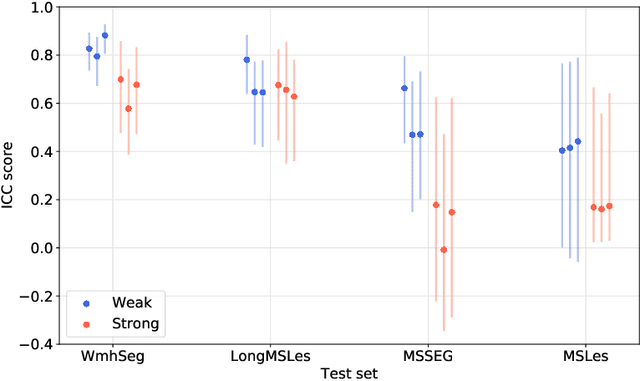
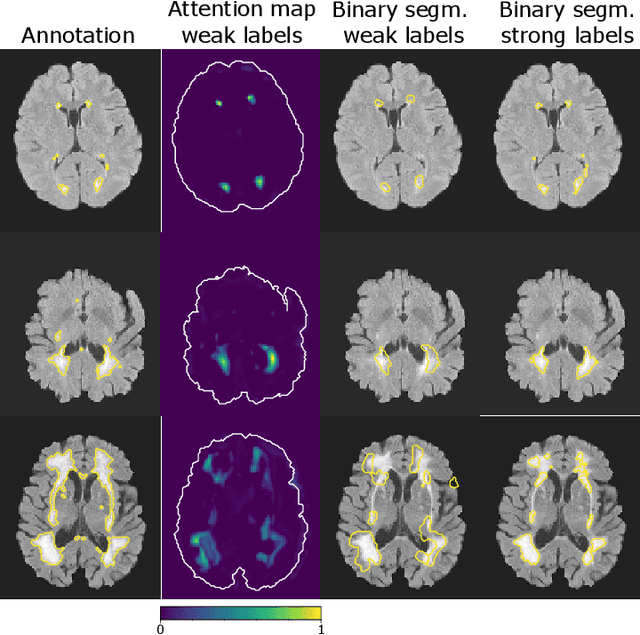
Abstract:To measure the volume of specific image structures, a typical approach is to first segment those structures using a neural network trained on voxel-wise (strong) labels and subsequently compute the volume from the segmentation. A more straightforward approach would be to predict the volume directly using a neural network based regression approach, trained on image-level (weak) labels indicating volume. In this article, we compared networks optimized with weak and strong labels, and study their ability to generalize to other datasets. We experimented with white matter hyperintensity (WMH) volume prediction in brain MRI scans. Neural networks were trained on a large local dataset and their performance was evaluated on four independent public datasets. We showed that networks optimized using only weak labels reflecting WMH volume generalized better for WMH volume prediction than networks optimized with voxel-wise segmentations of WMH. The attention maps of networks trained with weak labels did not seem to delineate WMHs, but highlighted instead areas with smooth contours around or near WMHs. By correcting for possible confounders we showed that networks trained on weak labels may have learnt other meaningful features that are more suited to generalization to unseen data. Our results suggest that for imaging biomarkers that can be derived from segmentations, training networks to predict the biomarker directly may provide more robust results than solving an intermediate segmentation step.
Automated Lesion Detection by Regressing Intensity-Based Distance with a Neural Network
Jul 29, 2019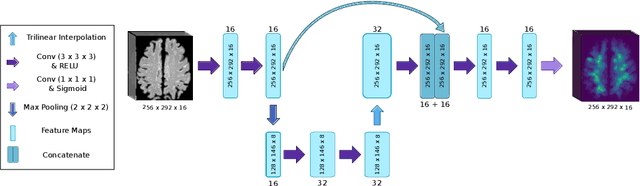

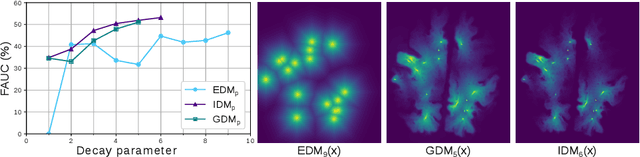
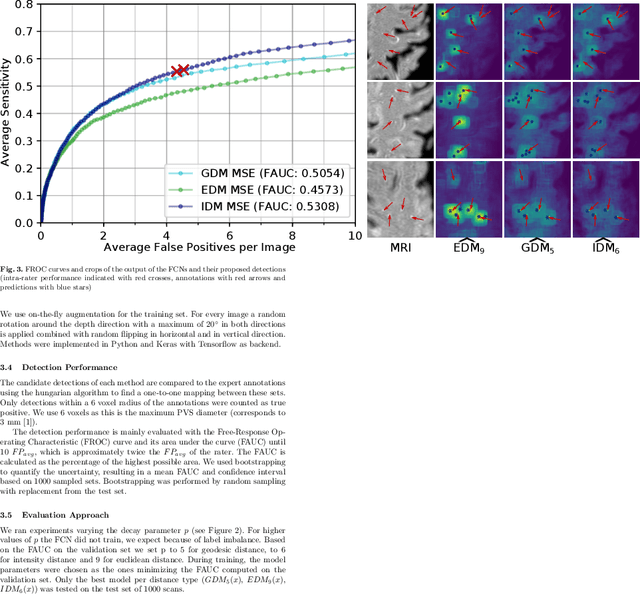
Abstract:Localization of focal vascular lesions on brain MRI is an important component of research on the etiology of neurological disorders. However, manual annotation of lesions can be challenging, time-consuming and subject to observer bias. Automated detection methods often need voxel-wise annotations for training. We propose a novel approach for automated lesion detection that can be trained on scans only annotated with a dot per lesion instead of a full segmentation. From the dot annotations and their corresponding intensity images we compute various distance maps (DMs), indicating the distance to a lesion based on spatial distance, intensity distance, or both. We train a fully convolutional neural network (FCN) to predict these DMs for unseen intensity images. The local optima in the predicted DMs are expected to correspond to lesion locations. We show the potential of this approach to detect enlarged perivascular spaces in white matter on a large brain MRI dataset with an independent test set of 1000 scans. Our method matches the intra-rater performance of the expert rater that was computed on an independent set. We compare the different types of distance maps, showing that incorporating intensity information in the distance maps used to train an FCN greatly improves performance.
Event-Based Modeling with High-Dimensional Imaging Biomarkers for Estimating Spatial Progression of Dementia
Mar 08, 2019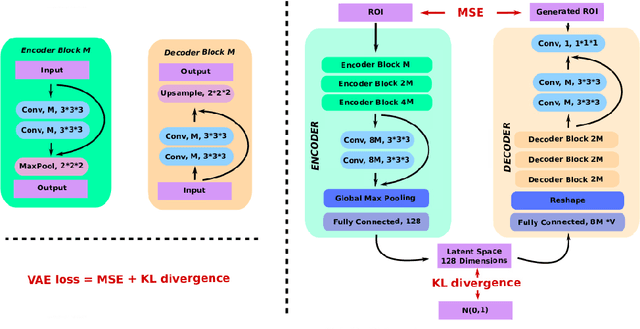
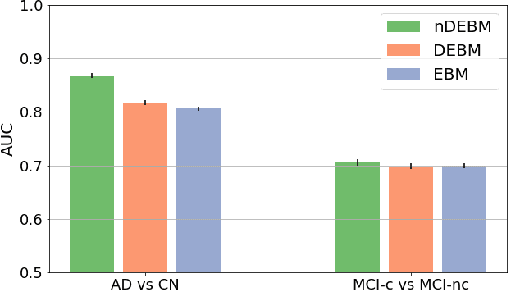
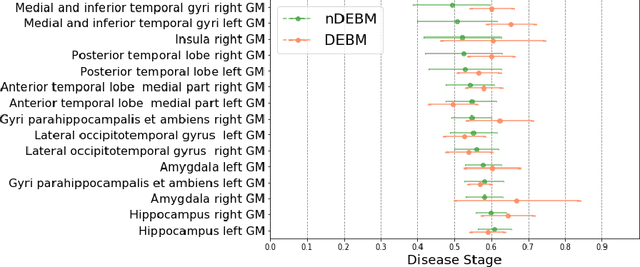
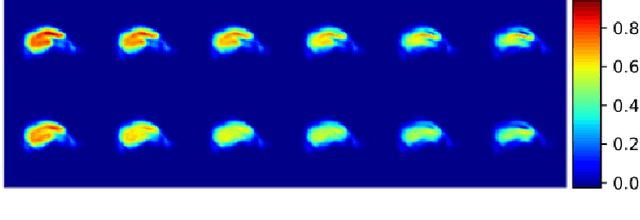
Abstract:Event-based models (EBM) are a class of disease progression models that can be used to estimate temporal ordering of neuropathological changes from cross-sectional data. Current EBMs only handle scalar biomarkers, such as regional volumes, as inputs. However, regional aggregates are a crude summary of the underlying high-resolution images, potentially limiting the accuracy of EBM. Therefore, we propose a novel method that exploits high-dimensional voxel-wise imaging biomarkers: n-dimensional discriminative EBM (nDEBM). nDEBM is based on an insight that mixture modeling, which is a key element of conventional EBMs, can be replaced by a more scalable semi-supervised support vector machine (SVM) approach. This SVM is used to estimate the degree of abnormality of each region which is then used to obtain subject-specific disease progression patterns. These patterns are in turn used for estimating the mean ordering by fitting a generalized Mallows model. In order to validate the biomarker ordering obtained using nDEBM, we also present a framework for Simulation of Imaging Biomarkers' Temporal Evolution (SImBioTE) that mimics neurodegeneration in brain regions. SImBioTE trains variational auto-encoders (VAE) in different brain regions independently to simulate images at varying stages of disease progression. We also validate nDEBM clinically using data from the Alzheimer's Disease Neuroimaging Initiative (ADNI). In both experiments, nDEBM using high-dimensional features gave better performance than state-of-the-art EBM methods using regional volume biomarkers. This suggests that nDEBM is a promising approach for disease progression modeling.
End-to-End Diagnosis and Segmentation Learning from Cardiac Magnetic Resonance Imaging
Oct 23, 2018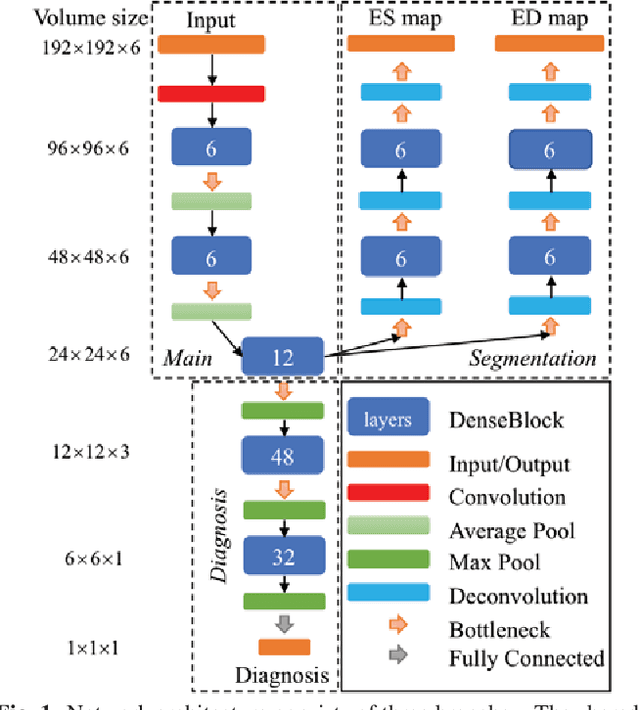
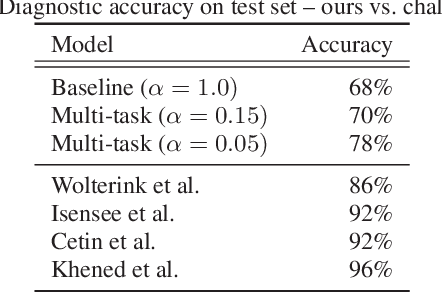
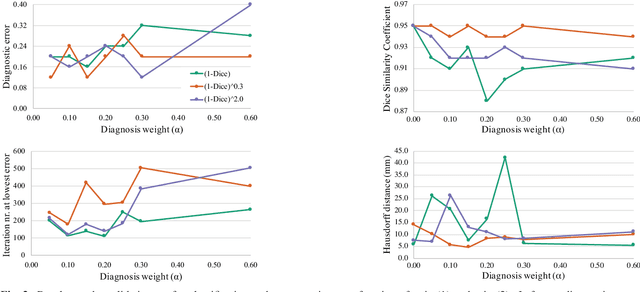
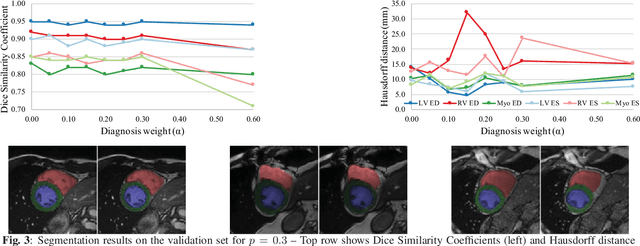
Abstract:Cardiac magnetic resonance (CMR) is used extensively in the diagnosis and management of cardiovascular disease. Deep learning methods have proven to deliver segmentation results comparable to human experts in CMR imaging, but there have been no convincing results for the problem of end-to-end segmentation and diagnosis from CMR. This is in part due to a lack of sufficiently large datasets required to train robust diagnosis models. In this paper, we propose a learning method to train diagnosis models, where our approach is designed to work with relatively small datasets. In particular, the optimisation loss is based on multi-task learning that jointly trains for the tasks of segmentation and diagnosis classification. We hypothesize that segmentation has a regularizing effect on the learning of features relevant for diagnosis. Using the 100 training and 50 testing samples available from the Automated Cardiac Diagnosis Challenge (ACDC) dataset, which has a balanced distribution of 5 cardiac diagnoses, we observe a reduction of the classification error from 32% to 22%, and a faster convergence compared to a baseline without segmentation. To the best of our knowledge, this is the best diagnosis results from CMR using an end-to-end diagnosis and segmentation learning method.
Disease Progression Timeline Estimation for Alzheimer's Disease using Discriminative Event Based Modeling
Aug 10, 2018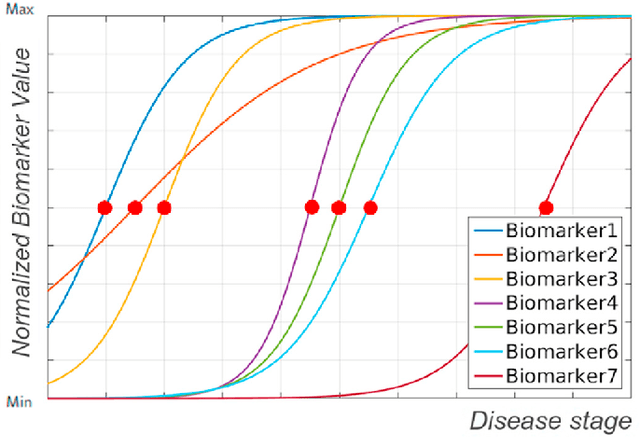

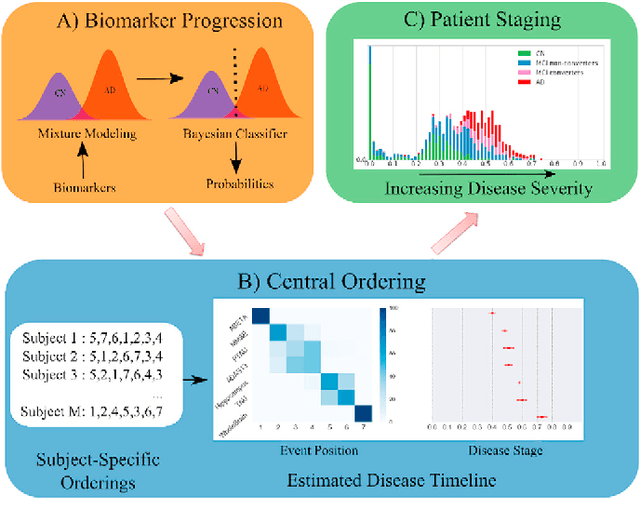
Abstract:Alzheimer's Disease (AD) is characterized by a cascade of biomarkers becoming abnormal, the pathophysiology of which is very complex and largely unknown. Event-based modeling (EBM) is a data-driven technique to estimate the sequence in which biomarkers for a disease become abnormal based on cross-sectional data. It can help in understanding the dynamics of disease progression and facilitate early diagnosis and prognosis. In this work we propose a novel discriminative approach to EBM, which is shown to be more accurate than existing state-of-the-art EBM methods. The method first estimates for each subject an approximate ordering of events. Subsequently, the central ordering over all subjects is estimated by fitting a generalized Mallows model to these approximate subject-specific orderings. We also introduce the concept of relative distance between events which helps in creating a disease progression timeline. Subsequently, we propose a method to stage subjects by placing them on the estimated disease progression timeline. We evaluated the proposed method on Alzheimer's Disease Neuroimaging Initiative (ADNI) data and compared the results with existing state-of-the-art EBM methods. We also performed extensive experiments on synthetic data simulating the progression of Alzheimer's disease. The event orderings obtained on ADNI data seem plausible and are in agreement with the current understanding of progression of AD. The proposed patient staging algorithm performed consistently better than that of state-of-the-art EBM methods. Event orderings obtained in simulation experiments were more accurate than those of other EBM methods and the estimated disease progression timeline was observed to correlate with the timeline of actual disease progression. The results of these experiments are encouraging and suggest that discriminative EBM is a promising approach to disease progression modeling.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge