Hong Bu
Department of Pathology, West China Hospital, Sichuan University, Chengdu, China, Laboratory of Pathology, Clinical Research Centre for Breast, West China Hospital, Sichuan University, Chengdu, China
Denoising Mutual Knowledge Distillation in Bi-Directional Multiple Instance Learning
May 17, 2025Abstract:Multiple Instance Learning is the predominant method for Whole Slide Image classification in digital pathology, enabling the use of slide-level labels to supervise model training. Although MIL eliminates the tedious fine-grained annotation process for supervised learning, whether it can learn accurate bag- and instance-level classifiers remains a question. To address the issue, instance-level classifiers and instance masks were incorporated to ground the prediction on supporting patches. These methods, while practically improving the performance of MIL methods, may potentially introduce noisy labels. We propose to bridge the gap between commonly used MIL and fully supervised learning by augmenting both the bag- and instance-level learning processes with pseudo-label correction capabilities elicited from weak to strong generalization techniques. The proposed algorithm improves the performance of dual-level MIL algorithms on both bag- and instance-level predictions. Experiments on public pathology datasets showcase the advantage of the proposed methods.
Patho-R1: A Multimodal Reinforcement Learning-Based Pathology Expert Reasoner
May 16, 2025



Abstract:Recent advances in vision language models (VLMs) have enabled broad progress in the general medical field. However, pathology still remains a more challenging subdomain, with current pathology specific VLMs exhibiting limitations in both diagnostic accuracy and reasoning plausibility. Such shortcomings are largely attributable to the nature of current pathology datasets, which are primarily composed of image description pairs that lack the depth and structured diagnostic paradigms employed by real world pathologists. In this study, we leverage pathology textbooks and real world pathology experts to construct high-quality, reasoning-oriented datasets. Building on this, we introduce Patho-R1, a multimodal RL-based pathology Reasoner, trained through a three-stage pipeline: (1) continued pretraining on 3.5 million image-text pairs for knowledge infusion; (2) supervised fine-tuning on 500k high-quality Chain-of-Thought samples for reasoning incentivizing; (3) reinforcement learning using Group Relative Policy Optimization and Decoupled Clip and Dynamic sAmpling Policy Optimization strategies for multimodal reasoning quality refinement. To further assess the alignment quality of our dataset, we propose PathoCLIP, trained on the same figure-caption corpus used for continued pretraining. Comprehensive experimental results demonstrate that both PathoCLIP and Patho-R1 achieve robust performance across a wide range of pathology-related tasks, including zero-shot classification, cross-modal retrieval, Visual Question Answering, and Multiple Choice Question. Our project is available at the Patho-R1 repository: https://github.com/Wenchuan-Zhang/Patho-R1.
Point Transformer with Federated Learning for Predicting Breast Cancer HER2 Status from Hematoxylin and Eosin-Stained Whole Slide Images
Dec 11, 2023Abstract:Directly predicting human epidermal growth factor receptor 2 (HER2) status from widely available hematoxylin and eosin (HE)-stained whole slide images (WSIs) can reduce technical costs and expedite treatment selection. Accurately predicting HER2 requires large collections of multi-site WSIs. Federated learning enables collaborative training of these WSIs without gigabyte-size WSIs transportation and data privacy concerns. However, federated learning encounters challenges in addressing label imbalance in multi-site WSIs from the real world. Moreover, existing WSI classification methods cannot simultaneously exploit local context information and long-range dependencies in the site-end feature representation of federated learning. To address these issues, we present a point transformer with federated learning for multi-site HER2 status prediction from HE-stained WSIs. Our approach incorporates two novel designs. We propose a dynamic label distribution strategy and an auxiliary classifier, which helps to establish a well-initialized model and mitigate label distribution variations across sites. Additionally, we propose a farthest cosine sampling based on cosine distance. It can sample the most distinctive features and capture the long-range dependencies. Extensive experiments and analysis show that our method achieves state-of-the-art performance at four sites with a total of 2687 WSIs. Furthermore, we demonstrate that our model can generalize to two unseen sites with 229 WSIs.
Validation of the Practicability of Logical Assessment Formula for Evaluations with Inaccurate Ground-Truth Labels
Jul 06, 2023Abstract:Logical assessment formula (LAF) is a new theory proposed for evaluations with inaccurate ground-truth labels (IAGTLs) to assess the predictive models for various artificial intelligence applications. However, the practicability of LAF for evaluations with IAGTLs has not yet been validated in real-world practice. In this paper, to address this issue, we applied LAF to tumour segmentation for breast cancer (TSfBC) in medical histopathology whole slide image analysis (MHWSIA). Experimental results and analysis show the validity of LAF for evaluations with IAGTLs in the case of TSfBC and reflect the potentials of LAF applied to MHWSIA.
Experts' cognition-driven ensemble deep learning for external validation of predicting pathological complete response to neoadjuvant chemotherapy from histological images in breast cancer
Jun 19, 2023Abstract:In breast cancer imaging, there has been a trend to directly predict pathological complete response (pCR) to neoadjuvant chemotherapy (NAC) from histological images based on deep learning (DL). However, it has been a commonly known problem that the constructed DL-based models numerically have better performances in internal validation than in external validation. The primary reason for this situation lies in that the distribution of the external data for validation is different from the distribution of the training data for the construction of the predictive model. In this paper, we aim to alleviate this situation with a more intrinsic approach. We propose an experts' cognition-driven ensemble deep learning (ECDEDL) approach for external validation of predicting pCR to NAC from histological images in breast cancer. The proposed ECDEDL, which takes the cognition of both pathology and artificial intelligence experts into consideration to improve the generalization of the predictive model to the external validation, more intrinsically approximates the working paradigm of a human being which will refer to his various working experiences to make decisions. The proposed ECDEDL approach was validated with 695 WSIs collected from the same center as the primary dataset to develop the predictive model and perform the internal validation, and 340 WSIs collected from other three centers as the external dataset to perform the external validation. In external validation, the proposed ECDEDL approach improves the AUCs of pCR prediction from 61.52(59.80-63.26) to 67.75(66.74-68.80) and the Accuracies of pCR prediction from 56.09(49.39-62.79) to 71.01(69.44-72.58). The proposed ECDEDL was quite effective for external validation, numerically more approximating the internal validation.
Experts' cognition-driven safe noisy labels learning for precise segmentation of residual tumor in breast cancer
Apr 13, 2023Abstract:Precise segmentation of residual tumor in breast cancer (PSRTBC) after neoadjuvant chemotherapy is a fundamental key technique in the treatment process of breast cancer. However, achieving PSRTBC is still a challenge, since the breast cancer tissue and tumor cells commonly have complex and varied morphological changes after neoadjuvant chemotherapy, which inevitably increases the difficulty to produce a predictive model that has good generalization with machine learning. To alleviate this situation, in this paper, we propose an experts' cognition-driven safe noisy labels learning (ECDSNLL) approach. In the concept of safe noisy labels learning, which is a typical type of safe weakly supervised learning, ECDSNLL is constructed by integrating the pathology experts' cognition about identifying residual tumor in breast cancer and the artificial intelligence experts' cognition about data modeling with provided data basis. We show the advantages of the proposed ECDSNLL approach and its promising potentials in addressing PSRTBC. We also release a better predictive model for achieving PSRTBC, which can be leveraged to promote the development of related application software.
Blind deblurring for microscopic pathology images using deep learning networks
Nov 24, 2020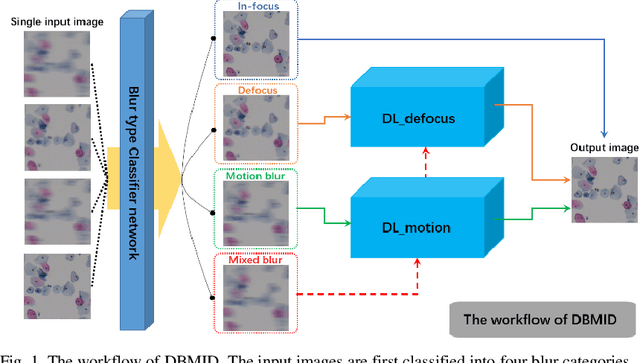
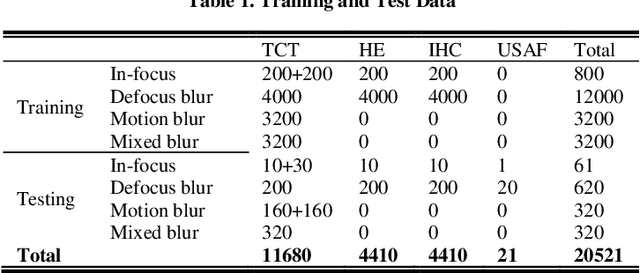
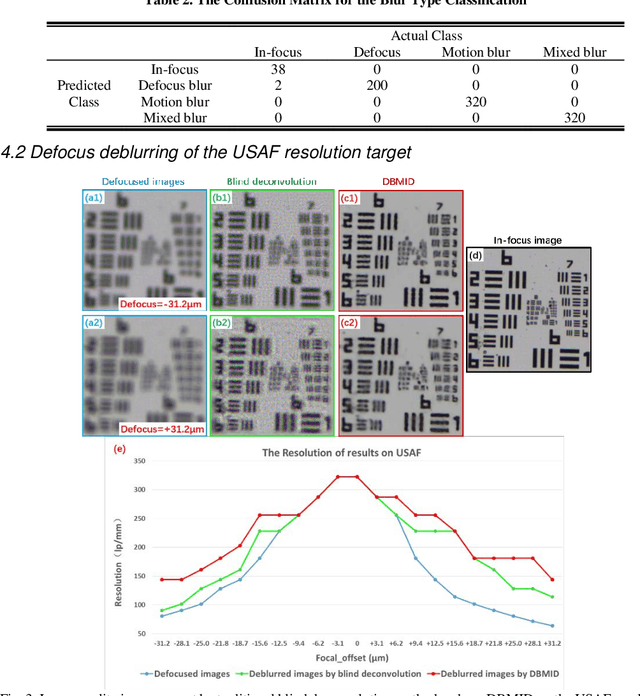
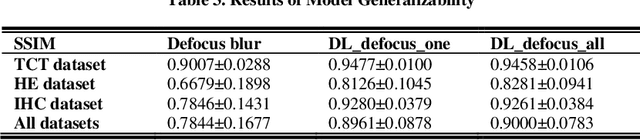
Abstract:Artificial Intelligence (AI)-powered pathology is a revolutionary step in the world of digital pathology and shows great promise to increase both diagnosis accuracy and efficiency. However, defocus and motion blur can obscure tissue or cell characteristics hence compromising AI algorithms'accuracy and robustness in analyzing the images. In this paper, we demonstrate a deep-learning-based approach that can alleviate the defocus and motion blur of a microscopic image and output a sharper and cleaner image with retrieved fine details without prior knowledge of the blur type, blur extent and pathological stain. In this approach, a deep learning classifier is first trained to identify the image blur type. Then, two encoder-decoder networks are trained and used alone or in combination to deblur the input image. It is an end-to-end approach and introduces no corrugated artifacts as traditional blind deconvolution methods do. We test our approach on different types of pathology specimens and demonstrate great performance on image blur correction and the subsequent improvement on the diagnosis outcome of AI algorithms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge