Feng Hou
Modality-Pairing Learning for Brain Tumor Segmentation
Oct 19, 2020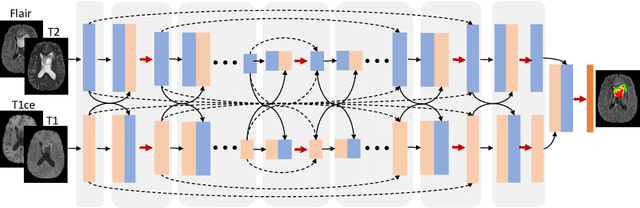
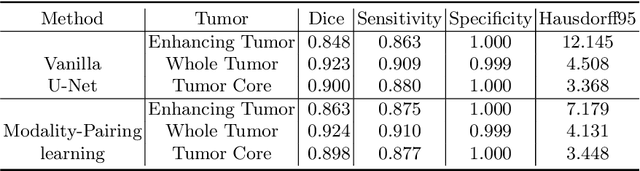
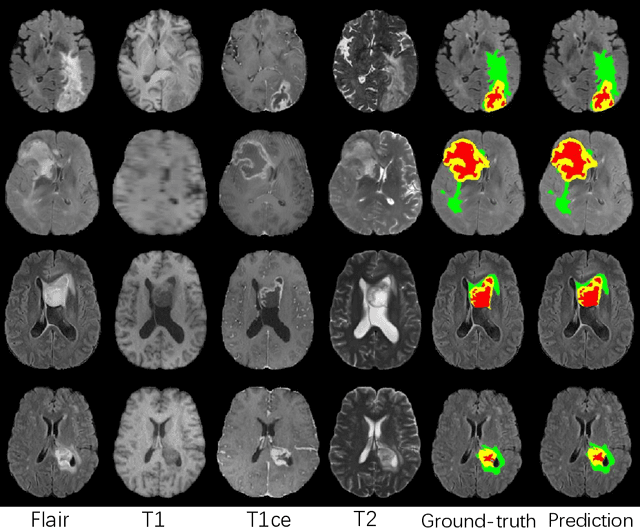
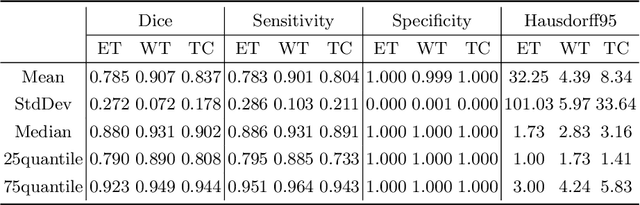
Abstract:Automatic brain tumor segmentation from multi-modality Magnetic Resonance Images (MRI) using deep learning methods plays an important role in assisting the diagnosis and treatment of brain tumor. However, previous methods mostly ignore the latent relationship among different modalities. In this work, we propose a novel end-to-end Modality-Pairing learning method for brain tumor segmentation. Paralleled branches are designed to exploit different modality features and a series of layer connections are utilized to capture complex relationships and abundant information among modalities. We also use a consistency loss to minimize the prediction variance between two branches. Besides, learning rate warmup strategy is adopted to solve the problem of the training instability and early over-fitting. Lastly, we use average ensemble of multiple models and some post-processing techniques to get final results. Our method is tested on the BraTS 2020 validation dataset, obtaining promising segmentation performance, with average dice scores of $0.908, 0.856, 0.787$ for the whole tumor, tumor core and enhancing tumor, respectively. We won the second place of the BraTS 2020 Challenge for the tumor segmentation on the testing dataset.
The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the KiTS19 Challenge
Dec 02, 2019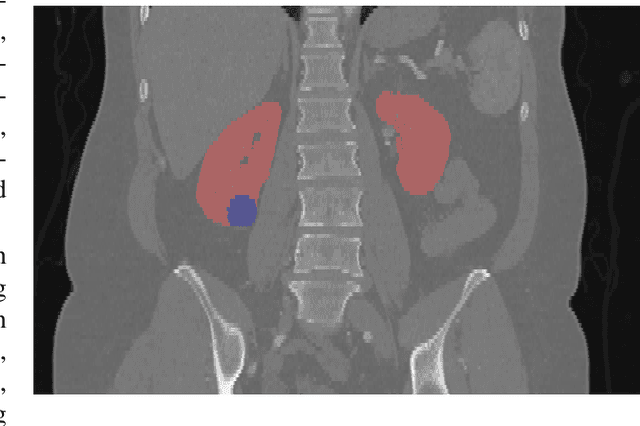
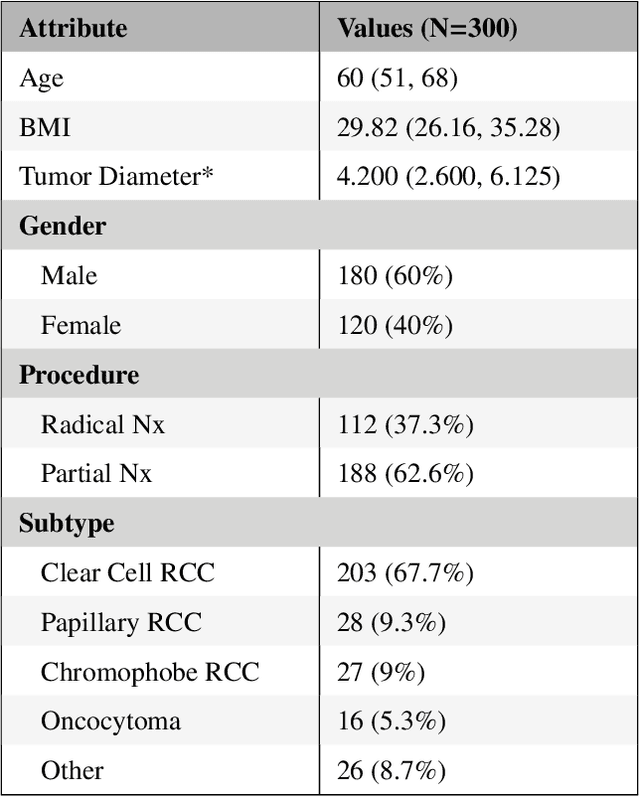
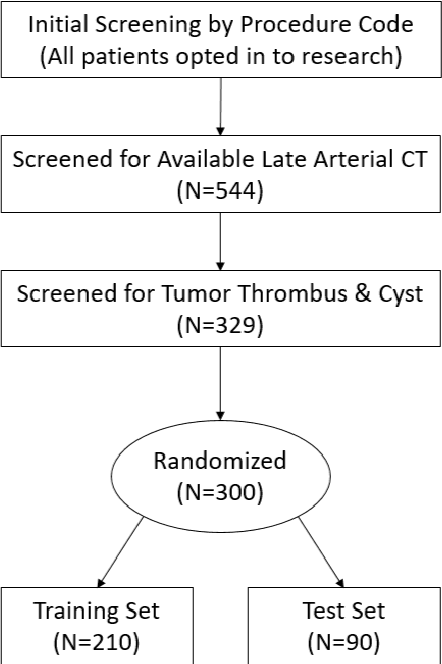
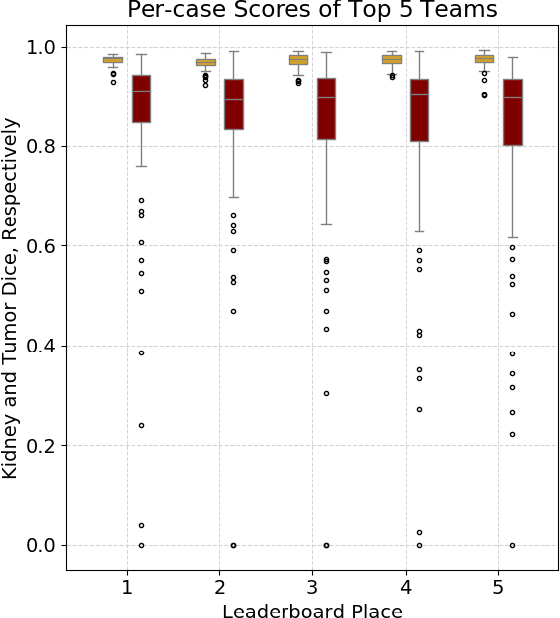
Abstract:There is a large body of literature linking anatomic and geometric characteristics of kidney tumors to perioperative and oncologic outcomes. Semantic segmentation of these tumors and their host kidneys is a promising tool for quantitatively characterizing these lesions, but its adoption is limited due to the manual effort required to produce high-quality 3D segmentations of these structures. Recently, methods based on deep learning have shown excellent results in automatic 3D segmentation, but they require large datasets for training, and there remains little consensus on which methods perform best. The 2019 Kidney and Kidney Tumor Segmentation challenge (KiTS19) was a competition held in conjunction with the 2019 International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI) which sought to address these issues and stimulate progress on this automatic segmentation problem. A training set of 210 cross sectional CT images with kidney tumors was publicly released with corresponding semantic segmentation masks. 106 teams from five continents used this data to develop automated systems to predict the true segmentation masks on a test set of 90 CT images for which the corresponding ground truth segmentations were kept private. These predictions were scored and ranked according to their average So rensen-Dice coefficient between the kidney and tumor across all 90 cases. The winning team achieved a Dice of 0.974 for kidney and 0.851 for tumor, approaching the inter-annotator performance on kidney (0.983) but falling short on tumor (0.923). This challenge has now entered an "open leaderboard" phase where it serves as a challenging benchmark in 3D semantic segmentation.
Cascaded Volumetric Convolutional Network for Kidney Tumor Segmentation from CT volumes
Oct 05, 2019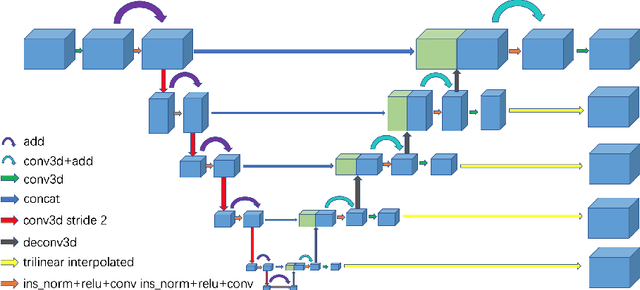
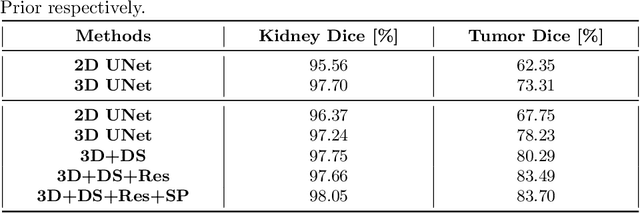
Abstract:Automated segmentation of kidney and tumor from 3D CT scans is necessary for the diagnosis, monitoring, and treatment planning of the disease. In this paper, we describe a two-stage framework for kidney and tumor segmentation based on 3D fully convolutional network (FCN). The first stage preliminarily locate the kidney and cut off the irrelevant background to reduce class imbalance and computation cost. Then the second stage precisely segment the kidney and tumor on the cropped patch. The proposed method achieves 98.05% and 83.70% of Dice score on the validation set of MICCAI 2019 KiTS Challenge.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge