Baiying Lei
Advanced Multimodal Learning for Seizure Detection and Prediction: Concept, Challenges, and Future Directions
Jan 08, 2026Abstract:Epilepsy is a chronic neurological disorder characterized by recurrent unprovoked seizures, affects over 50 million people worldwide, and poses significant risks, including sudden unexpected death in epilepsy (SUDEP). Conventional unimodal approaches, primarily reliant on electroencephalography (EEG), face several key challenges, including low SNR, nonstationarity, inter- and intrapatient heterogeneity, portability, and real-time applicability in clinical settings. To address these issues, a comprehensive survey highlights the concept of advanced multimodal learning for epileptic seizure detection and prediction (AMLSDP). The survey presents the evolution of epileptic seizure detection (ESD) and prediction (ESP) technologies across different eras. The survey also explores the core challenges of multimodal and non-EEG-based ESD and ESP. To overcome the key challenges of the multimodal system, the survey introduces the advanced processing strategies for efficient AMLSDP. Furthermore, this survey highlights future directions for researchers and practitioners. We believe this work will advance neurotechnology toward wearable and imaging-based solutions for epilepsy monitoring, serving as a valuable resource for future innovations in this domain.
BrainCSD: A Hierarchical Consistency-Driven MoE Foundation Model for Unified Connectome Synthesis and Multitask Brain Trait Prediction
Nov 07, 2025Abstract:Functional and structural connectivity (FC/SC) are key multimodal biomarkers for brain analysis, yet their clinical utility is hindered by costly acquisition, complex preprocessing, and frequent missing modalities. Existing foundation models either process single modalities or lack explicit mechanisms for cross-modal and cross-scale consistency. We propose BrainCSD, a hierarchical mixture-of-experts (MoE) foundation model that jointly synthesizes FC/SC biomarkers and supports downstream decoding tasks (diagnosis and prediction). BrainCSD features three neuroanatomically grounded components: (1) a ROI-specific MoE that aligns regional activations from canonical networks (e.g., DMN, FPN) with a global atlas via contrastive consistency; (2) a Encoding-Activation MOE that models dynamic cross-time/gradient dependencies in fMRI/dMRI; and (3) a network-aware refinement MoE that enforces structural priors and symmetry at individual and population levels. Evaluated on the datasets under complete and missing-modality settings, BrainCSD achieves SOTA results: 95.6\% accuracy for MCI vs. CN classification without FC, low synthesis error (FC RMSE: 0.038; SC RMSE: 0.006), brain age prediction (MAE: 4.04 years), and MMSE score estimation (MAE: 1.72 points). Code is available in \href{https://github.com/SXR3015/BrainCSD}{BrainCSD}
Pattern-Aware Diffusion Synthesis of fMRI/dMRI with Tissue and Microstructural Refinement
Nov 07, 2025Abstract:Magnetic resonance imaging (MRI), especially functional MRI (fMRI) and diffusion MRI (dMRI), is essential for studying neurodegenerative diseases. However, missing modalities pose a major barrier to their clinical use. Although GAN- and diffusion model-based approaches have shown some promise in modality completion, they remain limited in fMRI-dMRI synthesis due to (1) significant BOLD vs. diffusion-weighted signal differences between fMRI and dMRI in time/gradient axis, and (2) inadequate integration of disease-related neuroanatomical patterns during generation. To address these challenges, we propose PDS, introducing two key innovations: (1) a pattern-aware dual-modal 3D diffusion framework for cross-modality learning, and (2) a tissue refinement network integrated with a efficient microstructure refinement to maintain structural fidelity and fine details. Evaluated on OASIS-3, ADNI, and in-house datasets, our method achieves state-of-the-art results, with PSNR/SSIM scores of 29.83 dB/90.84\% for fMRI synthesis (+1.54 dB/+4.12\% over baselines) and 30.00 dB/77.55\% for dMRI synthesis (+1.02 dB/+2.2\%). In clinical validation, the synthesized data show strong diagnostic performance, achieving 67.92\%/66.02\%/64.15\% accuracy (NC vs. MCI vs. AD) in hybrid real-synthetic experiments. Code is available in \href{https://github.com/SXR3015/PDS}{PDS GitHub Repository}
Brain Structure-Function Fusing Representation Learning using Adversarial Decomposed-VAE for Analyzing MCI
May 23, 2023Abstract:Integrating the brain structural and functional connectivity features is of great significance in both exploring brain science and analyzing cognitive impairment clinically. However, it remains a challenge to effectively fuse structural and functional features in exploring the brain network. In this paper, a novel brain structure-function fusing-representation learning (BSFL) model is proposed to effectively learn fused representation from diffusion tensor imaging (DTI) and resting-state functional magnetic resonance imaging (fMRI) for mild cognitive impairment (MCI) analysis. Specifically, the decomposition-fusion framework is developed to first decompose the feature space into the union of the uniform and the unique spaces for each modality, and then adaptively fuse the decomposed features to learn MCI-related representation. Moreover, a knowledge-aware transformer module is designed to automatically capture local and global connectivity features throughout the brain. Also, a uniform-unique contrastive loss is further devised to make the decomposition more effective and enhance the complementarity of structural and functional features. The extensive experiments demonstrate that the proposed model achieves better performance than other competitive methods in predicting and analyzing MCI. More importantly, the proposed model could be a potential tool for reconstructing unified brain networks and predicting abnormal connections during the degenerative processes in MCI.
SG-GAN: Fine Stereoscopic-Aware Generation for 3D Brain Point Cloud Up-sampling from a Single Image
May 22, 2023Abstract:In minimally-invasive brain surgeries with indirect and narrow operating environments, 3D brain reconstruction is crucial. However, as requirements of accuracy for some new minimally-invasive surgeries (such as brain-computer interface surgery) are higher and higher, the outputs of conventional 3D reconstruction, such as point cloud (PC), are facing the challenges that sample points are too sparse and the precision is insufficient. On the other hand, there is a scarcity of high-density point cloud datasets, which makes it challenging to train models for direct reconstruction of high-density brain point clouds. In this work, a novel model named stereoscopic-aware graph generative adversarial network (SG-GAN) with two stages is proposed to generate fine high-density PC conditioned on a single image. The Stage-I GAN sketches the primitive shape and basic structure of the organ based on the given image, yielding Stage-I point clouds. The Stage-II GAN takes the results from Stage-I and generates high-density point clouds with detailed features. The Stage-II GAN is capable of correcting defects and restoring the detailed features of the region of interest (ROI) through the up-sampling process. Furthermore, a parameter-free-attention-based free-transforming module is developed to learn the efficient features of input, while upholding a promising performance. Comparing with the existing methods, the SG-GAN model shows superior performance in terms of visual quality, objective measurements, and performance in classification, as demonstrated by comprehensive results measured by several evaluation metrics including PC-to-PC error and Chamfer distance.
Brain Diffuser: An End-to-End Brain Image to Brain Network Pipeline
Mar 11, 2023Abstract:Brain network analysis is essential for diagnosing and intervention for Alzheimer's disease (AD). However, previous research relied primarily on specific time-consuming and subjective toolkits. Only few tools can obtain the structural brain networks from brain diffusion tensor images (DTI). In this paper, we propose a diffusion based end-to-end brain network generative model Brain Diffuser that directly shapes the structural brain networks from DTI. Compared to existing toolkits, Brain Diffuser exploits more structural connectivity features and disease-related information by analyzing disparities in structural brain networks across subjects. For the case of Alzheimer's disease, the proposed model performs better than the results from existing toolkits on the Alzheimer's Disease Neuroimaging Initiative (ADNI) database.
REFUGE2 Challenge: Treasure for Multi-Domain Learning in Glaucoma Assessment
Feb 24, 2022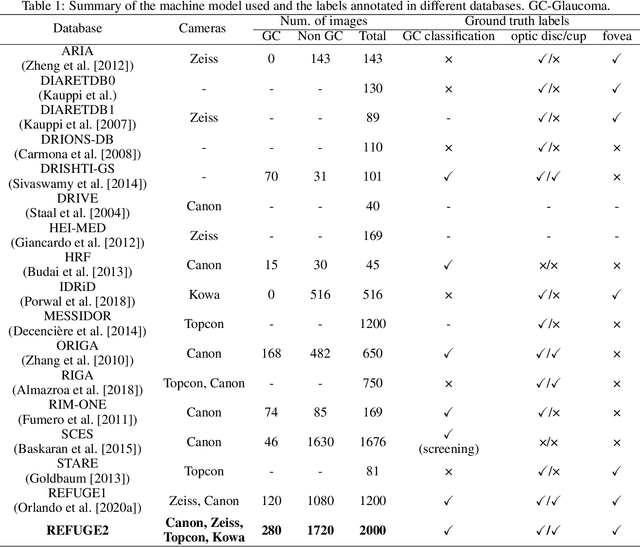
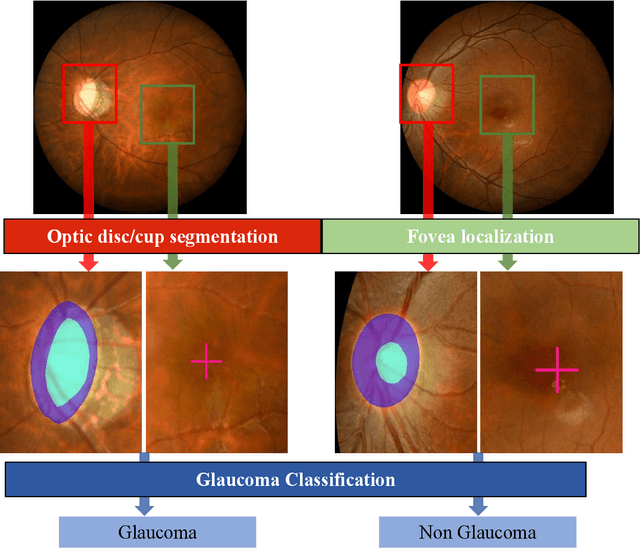
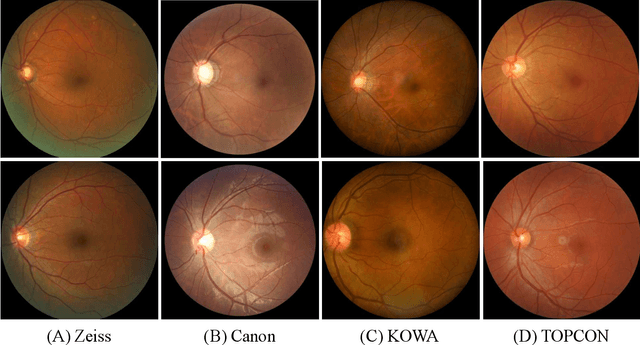
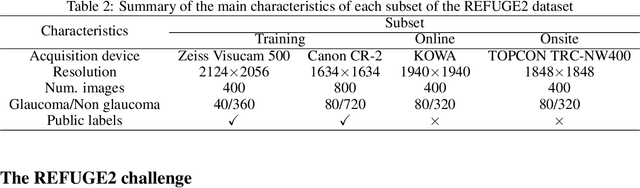
Abstract:Glaucoma is the second leading cause of blindness and is the leading cause of irreversible blindness disease in the world. Early screening for glaucoma in the population is significant. Color fundus photography is the most cost effective imaging modality to screen for ocular diseases. Deep learning network is often used in color fundus image analysis due to its powful feature extraction capability. However, the model training of deep learning method needs a large amount of data, and the distribution of data should be abundant for the robustness of model performance. To promote the research of deep learning in color fundus photography and help researchers further explore the clinical application signification of AI technology, we held a REFUGE2 challenge. This challenge released 2,000 color fundus images of four models, including Zeiss, Canon, Kowa and Topcon, which can validate the stabilization and generalization of algorithms on multi-domain. Moreover, three sub-tasks were designed in the challenge, including glaucoma classification, cup/optic disc segmentation, and macular fovea localization. These sub-tasks technically cover the three main problems of computer vision and clinicly cover the main researchs of glaucoma diagnosis. Over 1,300 international competitors joined the REFUGE2 challenge, 134 teams submitted more than 3,000 valid preliminary results, and 22 teams reached the final. This article summarizes the methods of some of the finalists and analyzes their results. In particular, we observed that the teams using domain adaptation strategies had high and robust performance on the dataset with multi-domain. This indicates that UDA and other multi-domain related researches will be the trend of deep learning field in the future, and our REFUGE2 datasets will play an important role in these researches.
ADAM Challenge: Detecting Age-related Macular Degeneration from Fundus Images
Feb 18, 2022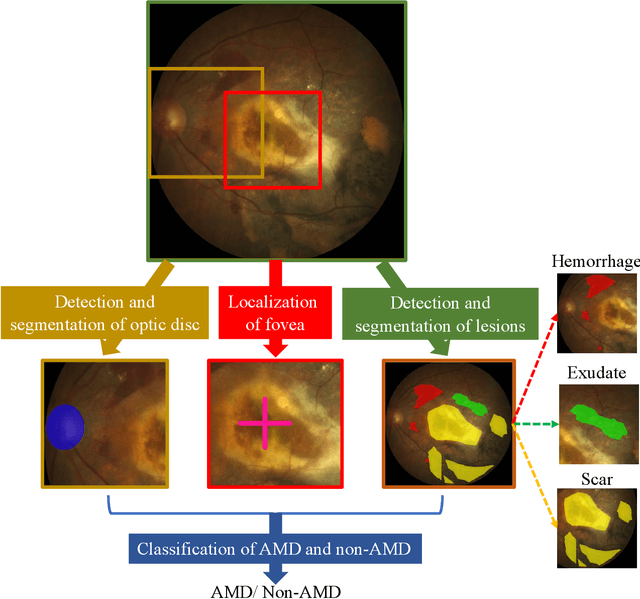
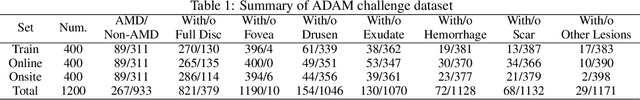
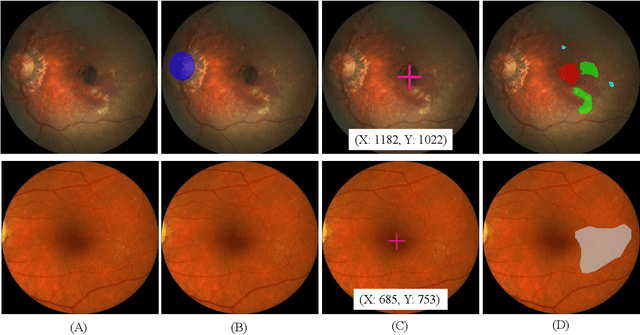
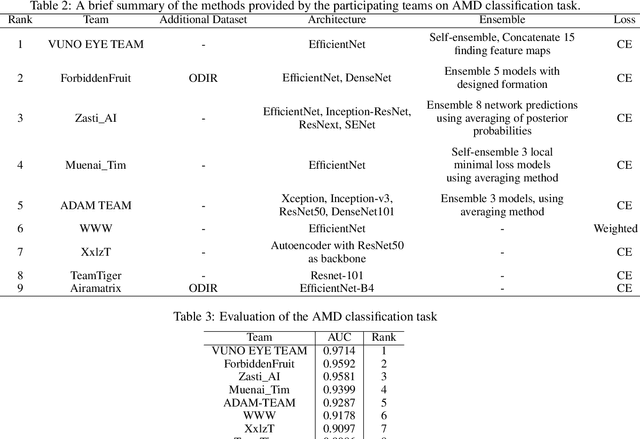
Abstract:Age-related macular degeneration (AMD) is the leading cause of visual impairment among elderly in the world. Early detection of AMD is of great importance as the vision loss caused by AMD is irreversible and permanent. Color fundus photography is the most cost-effective imaging modality to screen for retinal disorders. \textcolor{red}{Recently, some algorithms based on deep learning had been developed for fundus image analysis and automatic AMD detection. However, a comprehensive annotated dataset and a standard evaluation benchmark are still missing.} To deal with this issue, we set up the Automatic Detection challenge on Age-related Macular degeneration (ADAM) for the first time, held as a satellite event of the ISBI 2020 conference. The ADAM challenge consisted of four tasks which cover the main topics in detecting AMD from fundus images, including classification of AMD, detection and segmentation of optic disc, localization of fovea, and detection and segmentation of lesions. The ADAM challenge has released a comprehensive dataset of 1200 fundus images with the category labels of AMD, the pixel-wise segmentation masks of the full optic disc and lesions (drusen, exudate, hemorrhage, scar, and other), as well as the location coordinates of the macular fovea. A uniform evaluation framework has been built to make a fair comparison of different models. During the ADAM challenge, 610 results were submitted for online evaluation, and finally, 11 teams participated in the onsite challenge. This paper introduces the challenge, dataset, and evaluation methods, as well as summarizes the methods and analyzes the results of the participating teams of each task. In particular, we observed that ensembling strategy and clinical prior knowledge can better improve the performances of the deep learning models.
Morphological feature visualization of Alzheimer's disease via Multidirectional Perception GAN
Nov 25, 2021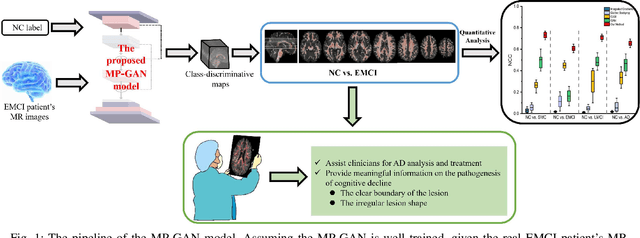
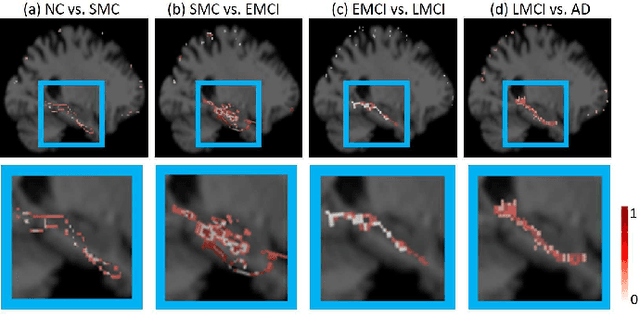
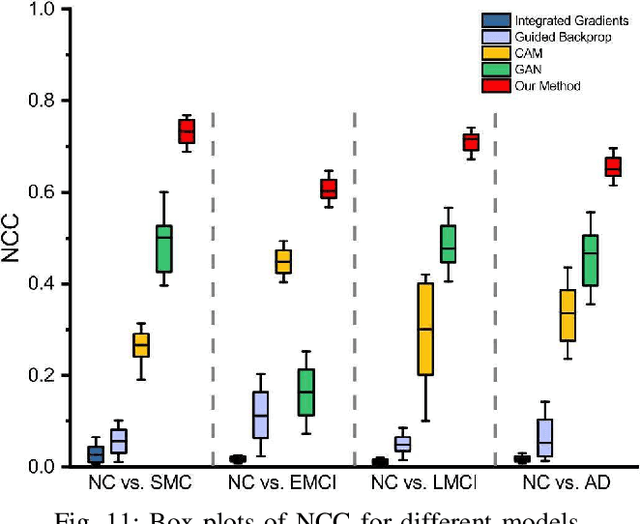
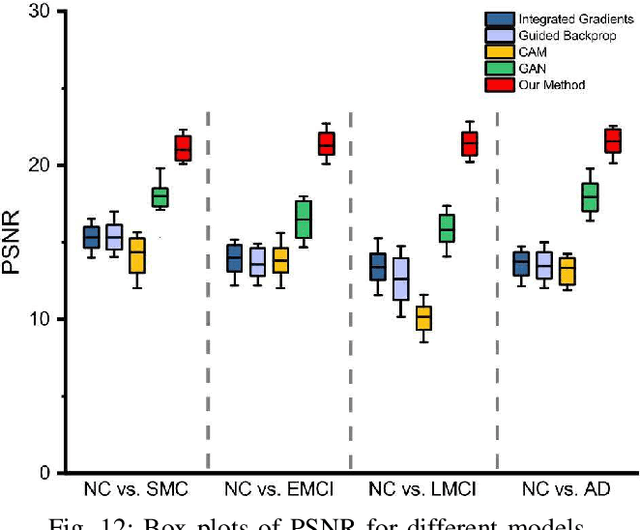
Abstract:The diagnosis of early stages of Alzheimer's disease (AD) is essential for timely treatment to slow further deterioration. Visualizing the morphological features for the early stages of AD is of great clinical value. In this work, a novel Multidirectional Perception Generative Adversarial Network (MP-GAN) is proposed to visualize the morphological features indicating the severity of AD for patients of different stages. Specifically, by introducing a novel multidirectional mapping mechanism into the model, the proposed MP-GAN can capture the salient global features efficiently. Thus, by utilizing the class-discriminative map from the generator, the proposed model can clearly delineate the subtle lesions via MR image transformations between the source domain and the pre-defined target domain. Besides, by integrating the adversarial loss, classification loss, cycle consistency loss and \emph{L}1 penalty, a single generator in MP-GAN can learn the class-discriminative maps for multiple-classes. Extensive experimental results on Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset demonstrate that MP-GAN achieves superior performance compared with the existing methods. The lesions visualized by MP-GAN are also consistent with what clinicians observe.
A Prior Guided Adversarial Representation Learning and Hypergraph Perceptual Network for Predicting Abnormal Connections of Alzheimer's Disease
Oct 12, 2021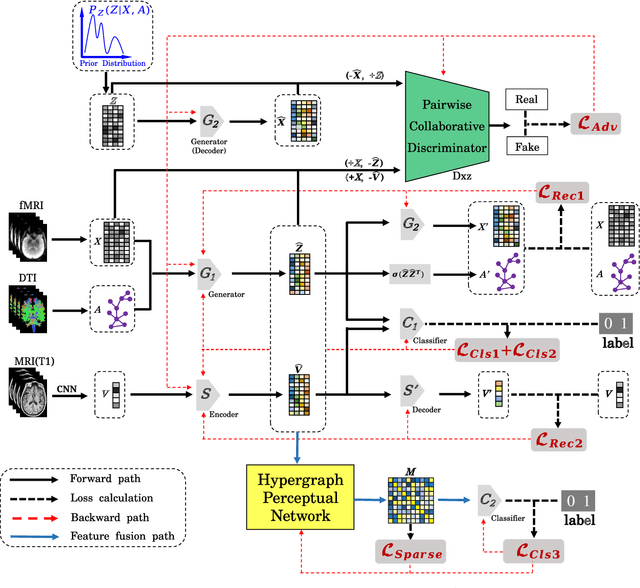
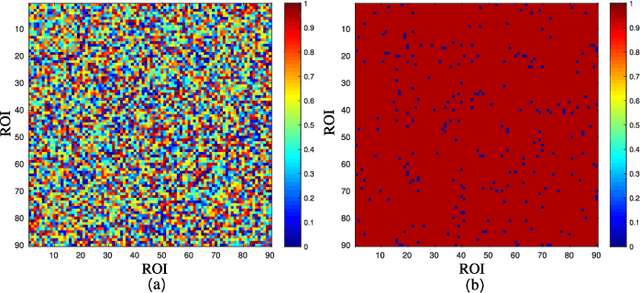
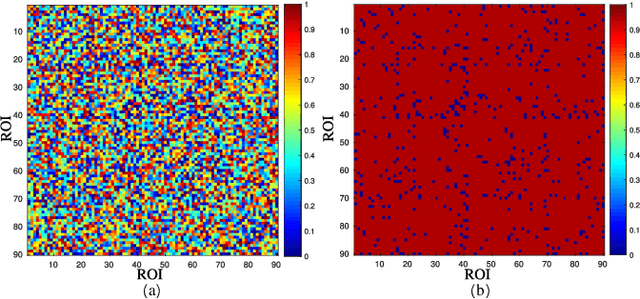
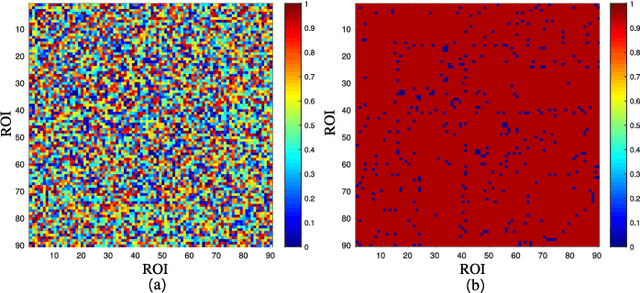
Abstract:Alzheimer's disease is characterized by alterations of the brain's structural and functional connectivity during its progressive degenerative processes. Existing auxiliary diagnostic methods have accomplished the classification task, but few of them can accurately evaluate the changing characteristics of brain connectivity. In this work, a prior guided adversarial representation learning and hypergraph perceptual network (PGARL-HPN) is proposed to predict abnormal brain connections using triple-modality medical images. Concretely, a prior distribution from the anatomical knowledge is estimated to guide multimodal representation learning using an adversarial strategy. Also, the pairwise collaborative discriminator structure is further utilized to narrow the difference of representation distribution. Moreover, the hypergraph perceptual network is developed to effectively fuse the learned representations while establishing high-order relations within and between multimodal images. Experimental results demonstrate that the proposed model outperforms other related methods in analyzing and predicting Alzheimer's disease progression. More importantly, the identified abnormal connections are partly consistent with the previous neuroscience discoveries. The proposed model can evaluate characteristics of abnormal brain connections at different stages of Alzheimer's disease, which is helpful for cognitive disease study and early treatment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge