cancer detection
Cancer detection using Artificial Intelligence (AI) involves leveraging advanced machine learning algorithms and techniques to identify and diagnose cancer from various medical data sources. The goal is to enhance early detection, improve diagnostic accuracy, and potentially reduce the need for invasive procedures.
Papers and Code
PolypSegTrack: Unified Foundation Model for Colonoscopy Video Analysis
Mar 31, 2025



Early detection, accurate segmentation, classification and tracking of polyps during colonoscopy are critical for preventing colorectal cancer. Many existing deep-learning-based methods for analyzing colonoscopic videos either require task-specific fine-tuning, lack tracking capabilities, or rely on domain-specific pre-training. In this paper, we introduce \textit{PolypSegTrack}, a novel foundation model that jointly addresses polyp detection, segmentation, classification and unsupervised tracking in colonoscopic videos. Our approach leverages a novel conditional mask loss, enabling flexible training across datasets with either pixel-level segmentation masks or bounding box annotations, allowing us to bypass task-specific fine-tuning. Our unsupervised tracking module reliably associates polyp instances across frames using object queries, without relying on any heuristics. We leverage a robust vision foundation model backbone that is pre-trained unsupervisedly on natural images, thereby removing the need for domain-specific pre-training. Extensive experiments on multiple polyp benchmarks demonstrate that our method significantly outperforms existing state-of-the-art approaches in detection, segmentation, classification, and tracking.
Hybrid CNN with Chebyshev Polynomial Expansion for Medical Image Analysis
Apr 09, 2025Lung cancer remains one of the leading causes of cancer-related mortality worldwide, with early and accurate diagnosis playing a pivotal role in improving patient outcomes. Automated detection of pulmonary nodules in computed tomography (CT) scans is a challenging task due to variability in nodule size, shape, texture, and location. Traditional Convolutional Neural Networks (CNNs) have shown considerable promise in medical image analysis; however, their limited ability to capture fine-grained spatial-spectral variations restricts their performance in complex diagnostic scenarios. In this study, we propose a novel hybrid deep learning architecture that incorporates Chebyshev polynomial expansions into CNN layers to enhance expressive power and improve the representation of underlying anatomical structures. The proposed Chebyshev-CNN leverages the orthogonality and recursive properties of Chebyshev polynomials to extract high-frequency features and approximate complex nonlinear functions with greater fidelity. The model is trained and evaluated on benchmark lung cancer imaging datasets, including LUNA16 and LIDC-IDRI, achieving superior performance in classifying pulmonary nodules as benign or malignant. Quantitative results demonstrate significant improvements in accuracy, sensitivity, and specificity compared to traditional CNN-based approaches. This integration of polynomial-based spectral approximation within deep learning provides a robust framework for enhancing automated medical diagnostics and holds potential for broader applications in clinical decision support systems.
Universal Lymph Node Detection in Multiparametric MRI with Selective Augmentation
Apr 07, 2025Robust localization of lymph nodes (LNs) in multiparametric MRI (mpMRI) is critical for the assessment of lymphadenopathy. Radiologists routinely measure the size of LN to distinguish benign from malignant nodes, which would require subsequent cancer staging. Sizing is a cumbersome task compounded by the diverse appearances of LNs in mpMRI, which renders their measurement difficult. Furthermore, smaller and potentially metastatic LNs could be missed during a busy clinical day. To alleviate these imaging and workflow problems, we propose a pipeline to universally detect both benign and metastatic nodes in the body for their ensuing measurement. The recently proposed VFNet neural network was employed to identify LN in T2 fat suppressed and diffusion weighted imaging (DWI) sequences acquired by various scanners with a variety of exam protocols. We also use a selective augmentation technique known as Intra-Label LISA (ILL) to diversify the input data samples the model sees during training, such that it improves its robustness during the evaluation phase. We achieved a sensitivity of $\sim$83\% with ILL vs. $\sim$80\% without ILL at 4 FP/vol. Compared with current LN detection approaches evaluated on mpMRI, we show a sensitivity improvement of $\sim$9\% at 4 FP/vol.
Automatic Prostate Volume Estimation in Transabdominal Ultrasound Images
Feb 11, 2025Prostate cancer is a leading health concern among men, requiring accurate and accessible methods for early detection and risk stratification. Prostate volume (PV) is a key parameter in multivariate risk stratification for early prostate cancer detection, commonly estimated using transrectal ultrasound (TRUS). While TRUS provides precise prostate volume measurements, its invasive nature often compromises patient comfort. Transabdominal ultrasound (TAUS) provides a non-invasive alternative but faces challenges such as lower image quality, complex interpretation, and reliance on operator expertise. This study introduces a new deep-learning-based framework for automatic PV estimation using TAUS, emphasizing its potential to enable accurate and non-invasive prostate cancer risk stratification. A dataset of TAUS videos from 100 individual patients was curated, with manually delineated prostate boundaries and calculated diameters by an expert clinician as ground truth. The introduced framework integrates deep-learning models for prostate segmentation in both axial and sagittal planes, automatic prostate diameter estimation, and PV calculation. Segmentation performance was evaluated using Dice correlation coefficient (%) and Hausdorff distance (mm). Framework's volume estimation capabilities were evaluated on volumetric error (mL). The framework demonstrates that it can estimate PV from TAUS videos with a mean volumetric error of -5.5 mL, which results in an average relative error between 5 and 15%. The introduced framework for automatic PV estimation from TAUS images, utilizing deep learning models for prostate segmentation, shows promising results. It effectively segments the prostate and estimates its volume, offering potential for reliable, non-invasive risk stratification for early prostate detection.
Opportunistic Screening for Pancreatic Cancer using Computed Tomography Imaging and Radiology Reports
Mar 31, 2025Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer, with most cases diagnosed at stage IV and a five-year overall survival rate below 5%. Early detection and prognosis modeling are crucial for improving patient outcomes and guiding early intervention strategies. In this study, we developed and evaluated a deep learning fusion model that integrates radiology reports and CT imaging to predict PDAC risk. The model achieved a concordance index (C-index) of 0.6750 (95% CI: 0.6429, 0.7121) and 0.6435 (95% CI: 0.6055, 0.6789) on the internal and external dataset, respectively, for 5-year survival risk estimation. Kaplan-Meier analysis demonstrated significant separation (p<0.0001) between the low and high risk groups predicted by the fusion model. These findings highlight the potential of deep learning-based survival models in leveraging clinical and imaging data for pancreatic cancer.
Evaluation of Vision Transformers for Multimodal Image Classification: A Case Study on Brain, Lung, and Kidney Tumors
Feb 08, 2025Neural networks have become the standard technique for medical diagnostics, especially in cancer detection and classification. This work evaluates the performance of Vision Transformers architectures, including Swin Transformer and MaxViT, in several datasets of magnetic resonance imaging (MRI) and computed tomography (CT) scans. We used three training sets of images with brain, lung, and kidney tumors. Each dataset includes different classification labels, from brain gliomas and meningiomas to benign and malignant lung conditions and kidney anomalies such as cysts and cancers. This work aims to analyze the behavior of the neural networks in each dataset and the benefits of combining different image modalities and tumor classes. We designed several experiments by fine-tuning the models on combined and individual image modalities. The results revealed that the Swin Transformer provided high accuracy, achieving up to 99.9\% for kidney tumor classification and 99.3\% accuracy in a combined dataset. MaxViT also provided excellent results in individual datasets but performed poorly when data is combined. This research highlights the adaptability of Transformer-based models to various image modalities and features. However, challenges persist, including limited annotated data and interpretability issues. Future works will expand this study by incorporating other image modalities and enhancing diagnostic capabilities. Integrating these models across diverse datasets could mark a pivotal advance in precision medicine, paving the way for more efficient and comprehensive healthcare solutions.
Automatic Robotic-Assisted Diffuse Reflectance Spectroscopy Scanning System
Mar 11, 2025



Diffuse Reflectance Spectroscopy (DRS) is a well-established optical technique for tissue composition assessment which has been clinically evaluated for tumour detection to ensure the complete removal of cancerous tissue. While point-wise assessment has many potential applications, incorporating automated large-area scanning would enable holistic tissue sampling with higher consistency. We propose a robotic system to facilitate autonomous DRS scanning with hybrid visual servoing control. A specially designed height compensation module enables precise contact condition control. The evaluation results show that the system can accurately execute the scanning command and acquire consistent DRS spectra with comparable results to the manual collection, which is the current gold standard protocol. Integrating the proposed system into surgery lays the groundwork for autonomous intra-operative DRS tissue assessment with high reliability and repeatability. This could reduce the need for manual scanning by the surgeon while ensuring complete tumor removal in clinical practice.
TransST: Transfer Learning Embedded Spatial Factor Modeling of Spatial Transcriptomics Data
Apr 15, 2025
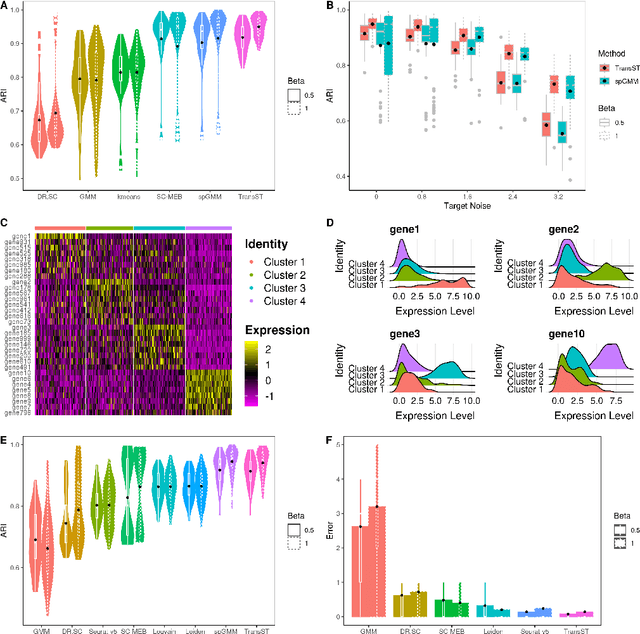


Background: Spatial transcriptomics have emerged as a powerful tool in biomedical research because of its ability to capture both the spatial contexts and abundance of the complete RNA transcript profile in organs of interest. However, limitations of the technology such as the relatively low resolution and comparatively insufficient sequencing depth make it difficult to reliably extract real biological signals from these data. To alleviate this challenge, we propose a novel transfer learning framework, referred to as TransST, to adaptively leverage the cell-labeled information from external sources in inferring cell-level heterogeneity of a target spatial transcriptomics data. Results: Applications in several real studies as well as a number of simulation settings show that our approach significantly improves existing techniques. For example, in the breast cancer study, TransST successfully identifies five biologically meaningful cell clusters, including the two subgroups of cancer in situ and invasive cancer; in addition, only TransST is able to separate the adipose tissues from the connective issues among all the studied methods. Conclusions: In summary, the proposed method TransST is both effective and robust in identifying cell subclusters and detecting corresponding driving biomarkers in spatial transcriptomics data.
Adaptive Voxel-Weighted Loss Using L1 Norms in Deep Neural Networks for Detection and Segmentation of Prostate Cancer Lesions in PET/CT Images
Feb 04, 2025



This study proposes a new loss function for deep neural networks, L1-weighted Dice Focal Loss (L1DFL), that leverages L1 norms for adaptive weighting of voxels based on their classification difficulty, towards automated detection and segmentation of metastatic prostate cancer lesions in PET/CT scans. We obtained 380 PSMA [18-F] DCFPyL PET/CT scans of patients diagnosed with biochemical recurrence metastatic prostate cancer. We trained two 3D convolutional neural networks, Attention U-Net and SegResNet, and concatenated the PET and CT volumes channel-wise as input. The performance of our custom loss function was evaluated against the Dice and Dice Focal Loss functions. For clinical significance, we considered a detected region of interest (ROI) as a true positive if at least the voxel with the maximum standardized uptake value falls within the ROI. We assessed the models' performance based on the number of lesions in an image, tumour volume, activity, and extent of spread. The L1DFL outperformed the comparative loss functions by at least 13% on the test set. In addition, the F1 scores of the Dice Loss and the Dice Focal Loss were lower than that of L1DFL by at least 6% and 34%, respectively. The Dice Focal Loss yielded more false positives, whereas the Dice Loss was more sensitive to smaller volumes and struggled to segment larger lesions accurately. They also exhibited network-specific variations and yielded declines in segmentation accuracy with increased tumour spread. Our results demonstrate the potential of L1DFL to yield robust segmentation of metastatic prostate cancer lesions in PSMA PET/CT images. The results further highlight potential complexities arising from the variations in lesion characteristics that may influence automated prostate cancer tumour detection and segmentation. The code is publicly available at: https://github.com/ObedDzik/pca_segment.git.
Safety-Ensured Control Framework for Robotic Endoscopic Task Automation
Mar 11, 2025



There is growing interest in automating surgical tasks using robotic systems, such as endoscopy for treating gastrointestinal (GI) cancer. However, previous studies have primarily focused on detecting and analyzing objects or robots, with limited attention to ensuring safety, which is critical for clinical applications, where accidents can be caused by unsafe robot motions. In this study, we propose a new control framework that can formally ensure the safety of automating certain processes involved in endoscopic submucosal dissection (ESD), a representative endoscopic surgical method for the treatment of early GI cancer, by using an endoscopic robot. The proposed framework utilizes Control Barrier Functions (CBFs) to accurately identify the boundaries of individual tumors, even in close proximity within the GI tract, ensuring precise treatment and removal while preserving the surrounding normal tissue. Additionally, by adopting a model-free control scheme, safety assurance is made possible even in endoscopic robotic systems where dynamic modeling is challenging. We demonstrate the proposed framework in cases where the tumors to be removed are close to each other, showing that the safety constraints are enforced. We show that the model-free CBF-based controlled robot eliminates one tumor completely without damaging it, while not invading another nearby tumor.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge