Paul M. Thompson
for the Alzheimer's Disease Neuroimaging Initiative
Classification of Major Depressive Disorder via Multi-Site Weighted LASSO Model
Jun 03, 2017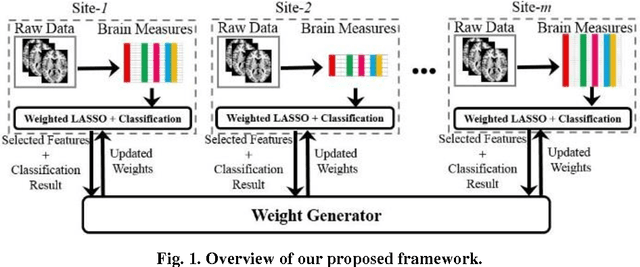
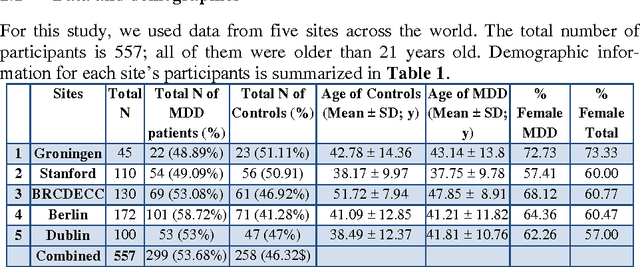
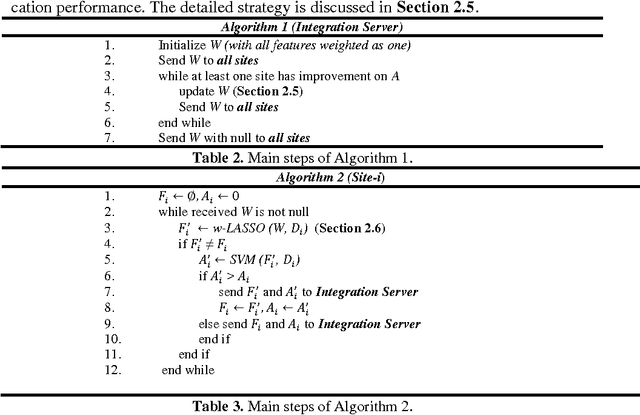
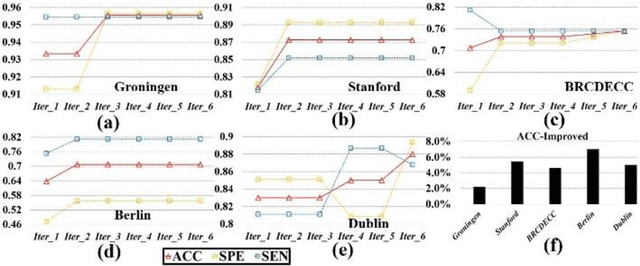
Abstract:Large-scale collaborative analysis of brain imaging data, in psychiatry and neu-rology, offers a new source of statistical power to discover features that boost ac-curacy in disease classification, differential diagnosis, and outcome prediction. However, due to data privacy regulations or limited accessibility to large datasets across the world, it is challenging to efficiently integrate distributed information. Here we propose a novel classification framework through multi-site weighted LASSO: each site performs an iterative weighted LASSO for feature selection separately. Within each iteration, the classification result and the selected features are collected to update the weighting parameters for each feature. This new weight is used to guide the LASSO process at the next iteration. Only the fea-tures that help to improve the classification accuracy are preserved. In tests on da-ta from five sites (299 patients with major depressive disorder (MDD) and 258 normal controls), our method boosted classification accuracy for MDD by 4.9% on average. This result shows the potential of the proposed new strategy as an ef-fective and practical collaborative platform for machine learning on large scale distributed imaging and biobank data.
Large-scale Feature Selection of Risk Genetic Factors for Alzheimer's Disease via Distributed Group Lasso Regression
Apr 27, 2017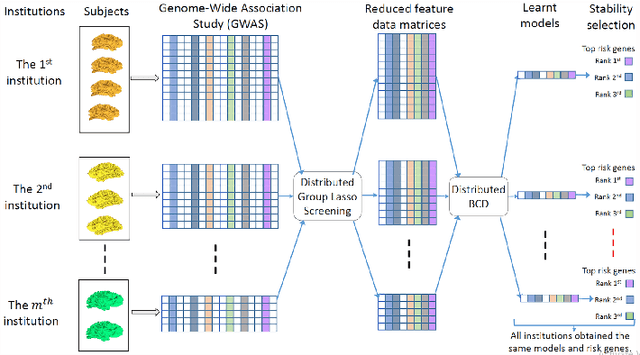
Abstract:Genome-wide association studies (GWAS) have achieved great success in the genetic study of Alzheimer's disease (AD). Collaborative imaging genetics studies across different research institutions show the effectiveness of detecting genetic risk factors. However, the high dimensionality of GWAS data poses significant challenges in detecting risk SNPs for AD. Selecting relevant features is crucial in predicting the response variable. In this study, we propose a novel Distributed Feature Selection Framework (DFSF) to conduct the large-scale imaging genetics studies across multiple institutions. To speed up the learning process, we propose a family of distributed group Lasso screening rules to identify irrelevant features and remove them from the optimization. Then we select the relevant group features by performing the group Lasso feature selection process in a sequence of parameters. Finally, we employ the stability selection to rank the top risk SNPs that might help detect the early stage of AD. To the best of our knowledge, this is the first distributed feature selection model integrated with group Lasso feature selection as well as detecting the risk genetic factors across multiple research institutions system. Empirical studies are conducted on 809 subjects with 5.9 million SNPs which are distributed across several individual institutions, demonstrating the efficiency and effectiveness of the proposed method.
A Restaurant Process Mixture Model for Connectivity Based Parcellation of the Cortex
Mar 02, 2017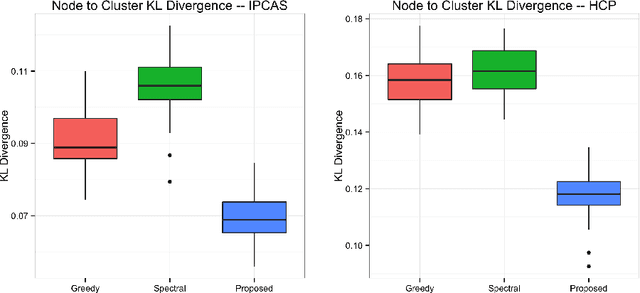
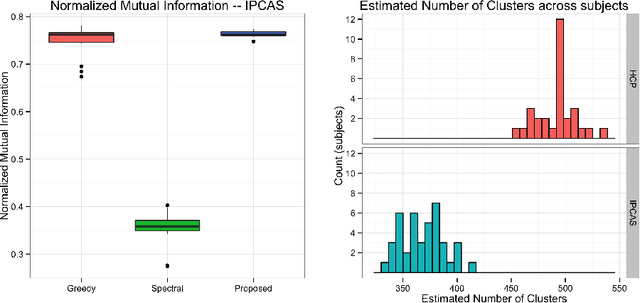
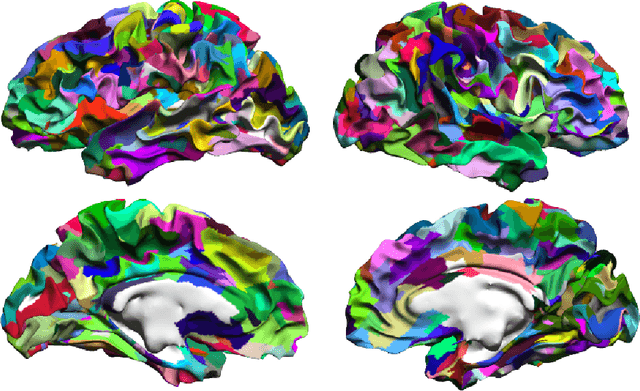
Abstract:One of the primary objectives of human brain mapping is the division of the cortical surface into functionally distinct regions, i.e. parcellation. While it is generally agreed that at macro-scale different regions of the cortex have different functions, the exact number and configuration of these regions is not known. Methods for the discovery of these regions are thus important, particularly as the volume of available information grows. Towards this end, we present a parcellation method based on a Bayesian non-parametric mixture model of cortical connectivity.
An Empirical Study of Continuous Connectivity Degree Sequence Equivalents
Nov 18, 2016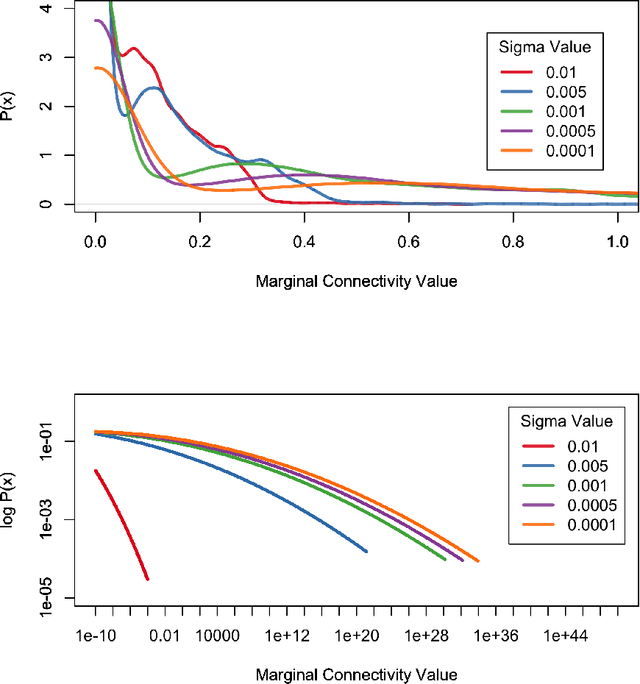
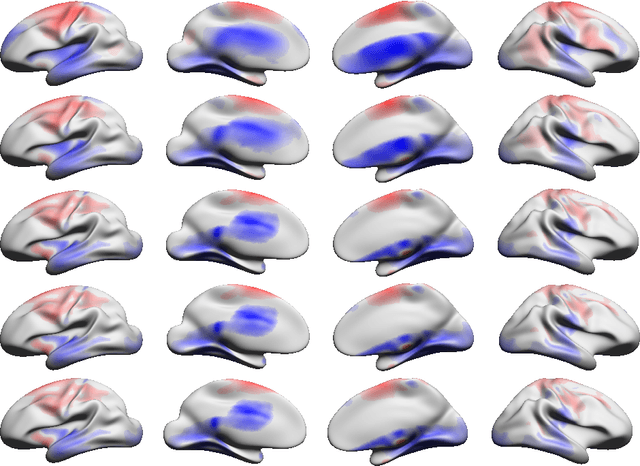
Abstract:In the present work we demonstrate the use of a parcellation free connectivity model based on Poisson point processes. This model produces for each subject a continuous bivariate intensity function that represents for every possible pair of points the relative rate at which we observe tracts terminating at those points. We fit this model to explore degree sequence equivalents for spatial continuum graphs, and to investigate the local differences between estimated intensity functions for two different tractography methods. This is a companion paper to Moyer et al. (2016), where the model was originally defined.
Large-scale Collaborative Imaging Genetics Studies of Risk Genetic Factors for Alzheimer's Disease Across Multiple Institutions
Aug 19, 2016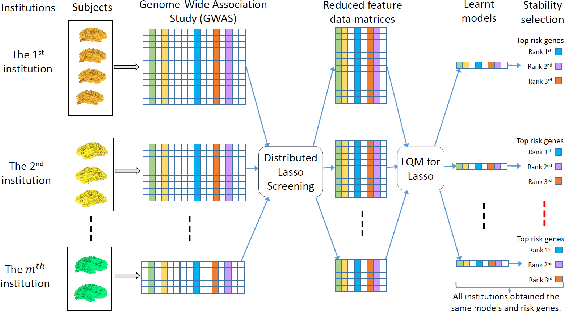
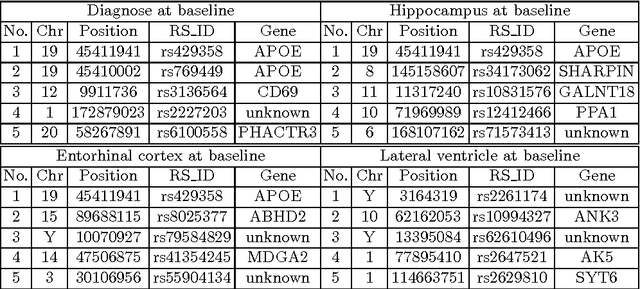
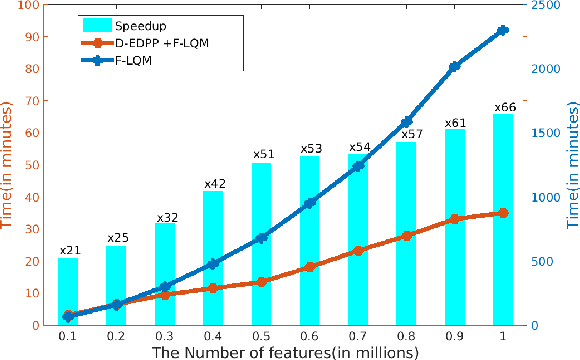
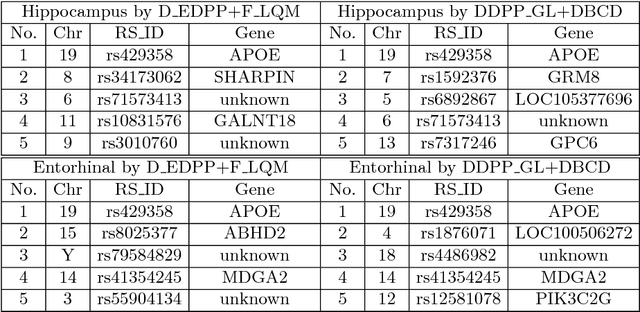
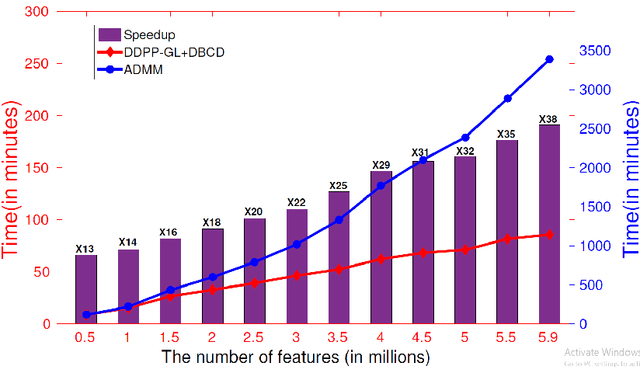
Abstract:Genome-wide association studies (GWAS) offer new opportunities to identify genetic risk factors for Alzheimer's disease (AD). Recently, collaborative efforts across different institutions emerged that enhance the power of many existing techniques on individual institution data. However, a major barrier to collaborative studies of GWAS is that many institutions need to preserve individual data privacy. To address this challenge, we propose a novel distributed framework, termed Local Query Model (LQM) to detect risk SNPs for AD across multiple research institutions. To accelerate the learning process, we propose a Distributed Enhanced Dual Polytope Projection (D-EDPP) screening rule to identify irrelevant features and remove them from the optimization. To the best of our knowledge, this is the first successful run of the computationally intensive model selection procedure to learn a consistent model across different institutions without compromising their privacy while ranking the SNPs that may collectively affect AD. Empirical studies are conducted on 809 subjects with 5.9 million SNP features which are distributed across three individual institutions. D-EDPP achieved a 66-fold speed-up by effectively identifying irrelevant features.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge