Sujay Kakarmath
Yifan
LLM-based Text Simplification and its Effect on User Comprehension and Cognitive Load
May 04, 2025Abstract:Information on the web, such as scientific publications and Wikipedia, often surpasses users' reading level. To help address this, we used a self-refinement approach to develop a LLM capability for minimally lossy text simplification. To validate our approach, we conducted a randomized study involving 4563 participants and 31 texts spanning 6 broad subject areas: PubMed (biomedical scientific articles), biology, law, finance, literature/philosophy, and aerospace/computer science. Participants were randomized to viewing original or simplified texts in a subject area, and answered multiple-choice questions (MCQs) that tested their comprehension of the text. The participants were also asked to provide qualitative feedback such as task difficulty. Our results indicate that participants who read the simplified text answered more MCQs correctly than their counterparts who read the original text (3.9% absolute increase, p<0.05). This gain was most striking with PubMed (14.6%), while more moderate gains were observed for finance (5.5%), aerospace/computer science (3.8%) domains, and legal (3.5%). Notably, the results were robust to whether participants could refer back to the text while answering MCQs. The absolute accuracy decreased by up to ~9% for both original and simplified setups where participants could not refer back to the text, but the ~4% overall improvement persisted. Finally, participants' self-reported perceived ease based on a simplified NASA Task Load Index was greater for those who read the simplified text (absolute change on a 5-point scale 0.33, p<0.05). This randomized study, involving an order of magnitude more participants than prior works, demonstrates the potential of LLMs to make complex information easier to understand. Our work aims to enable a broader audience to better learn and make use of expert knowledge available on the web, improving information accessibility.
HeAR -- Health Acoustic Representations
Mar 04, 2024



Abstract:Health acoustic sounds such as coughs and breaths are known to contain useful health signals with significant potential for monitoring health and disease, yet are underexplored in the medical machine learning community. The existing deep learning systems for health acoustics are often narrowly trained and evaluated on a single task, which is limited by data and may hinder generalization to other tasks. To mitigate these gaps, we develop HeAR, a scalable self-supervised learning-based deep learning system using masked autoencoders trained on a large dataset of 313 million two-second long audio clips. Through linear probes, we establish HeAR as a state-of-the-art health audio embedding model on a benchmark of 33 health acoustic tasks across 6 datasets. By introducing this work, we hope to enable and accelerate further health acoustics research.
Predicting Cardiovascular Disease Risk using Photoplethysmography and Deep Learning
May 09, 2023Abstract:Cardiovascular diseases (CVDs) are responsible for a large proportion of premature deaths in low- and middle-income countries. Early CVD detection and intervention is critical in these populations, yet many existing CVD risk scores require a physical examination or lab measurements, which can be challenging in such health systems due to limited accessibility. Here we investigated the potential to use photoplethysmography (PPG), a sensing technology available on most smartphones that can potentially enable large-scale screening at low cost, for CVD risk prediction. We developed a deep learning PPG-based CVD risk score (DLS) to predict the probability of having major adverse cardiovascular events (MACE: non-fatal myocardial infarction, stroke, and cardiovascular death) within ten years, given only age, sex, smoking status and PPG as predictors. We compared the DLS with the office-based refit-WHO score, which adopts the shared predictors from WHO and Globorisk scores (age, sex, smoking status, height, weight and systolic blood pressure) but refitted on the UK Biobank (UKB) cohort. In UKB cohort, DLS's C-statistic (71.1%, 95% CI 69.9-72.4) was non-inferior to office-based refit-WHO score (70.9%, 95% CI 69.7-72.2; non-inferiority margin of 2.5%, p<0.01). The calibration of the DLS was satisfactory, with a 1.8% mean absolute calibration error. Adding DLS features to the office-based score increased the C-statistic by 1.0% (95% CI 0.6-1.4). DLS predicts ten-year MACE risk comparable with the office-based refit-WHO score. It provides a proof-of-concept and suggests the potential of a PPG-based approach strategies for community-based primary prevention in resource-limited regions.
Deploying clinical machine learning? Consider the following...
Sep 14, 2021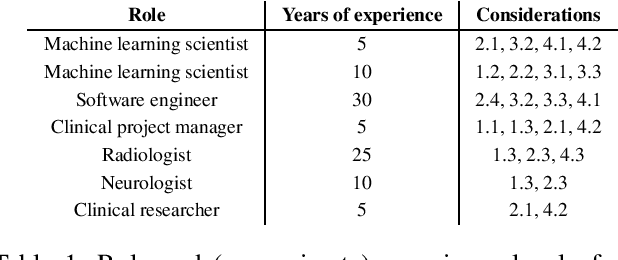
Abstract:Despite the intense attention and investment into clinical machine learning (CML) research, relatively few applications convert to clinical practice. While research is important in advancing the state-of-the-art, translation is equally important in bringing these technologies into a position to ultimately impact patient care and live up to extensive expectations surrounding AI in healthcare. To better characterize a holistic perspective among researchers and practitioners, we survey several participants with experience in developing CML for clinical deployment about their learned experiences. We collate these insights and identify several main categories of barriers and pitfalls in order to better design and develop clinical machine learning applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge