Siegfried K. Wagner
oculomix: Hierarchical Sampling for Retinal-Based Systemic Disease Prediction
Jan 16, 2026Abstract:Oculomics - the concept of predicting systemic diseases, such as cardiovascular disease and dementia, through retinal imaging - has advanced rapidly due to the data efficiency of transformer-based foundation models like RETFound. Image-level mixed sample data augmentations, such as CutMix and MixUp, are frequently used for training transformers, yet these techniques perturb patient-specific attributes, such as medical comorbidity and clinical factors, since they only account for images and labels. To address this limitation, we propose a hierarchical sampling strategy, Oculomix, for mixed sample augmentations. Our method is based on two clinical priors. First (exam level), images acquired from the same patient at the same time point share the same attributes. Second (patient level), images acquired from the same patient at different time points have a soft temporal trend, as morbidity generally increases over time. Guided by these priors, our method constrains the mixing space to the patient and exam levels to better preserve patient-specific characteristics and leverages their hierarchical relationships. The proposed method is validated using ViT models on a five-year prediction of major adverse cardiovascular events (MACE) in a large ethnically diverse population (Alzeye). We show that Oculomix consistently outperforms image-level CutMix and MixUp by up to 3% in AUROC, demonstrating the necessity and value of the proposed method in oculomics.
Generalist versus Specialist Vision Foundation Models for Ocular Disease and Oculomics
Sep 03, 2025Abstract:Medical foundation models, pre-trained with large-scale clinical data, demonstrate strong performance in diverse clinically relevant applications. RETFound, trained on nearly one million retinal images, exemplifies this approach in applications with retinal images. However, the emergence of increasingly powerful and multifold larger generalist foundation models such as DINOv2 and DINOv3 raises the question of whether domain-specific pre-training remains essential, and if so, what gap persists. To investigate this, we systematically evaluated the adaptability of DINOv2 and DINOv3 in retinal image applications, compared to two specialist RETFound models, RETFound-MAE and RETFound-DINOv2. We assessed performance on ocular disease detection and systemic disease prediction using two adaptation strategies: fine-tuning and linear probing. Data efficiency and adaptation efficiency were further analysed to characterise trade-offs between predictive performance and computational cost. Our results show that although scaling generalist models yields strong adaptability across diverse tasks, RETFound-DINOv2 consistently outperforms these generalist foundation models in ocular-disease detection and oculomics tasks, demonstrating stronger generalisability and data efficiency. These findings suggest that specialist retinal foundation models remain the most effective choice for clinical applications, while the narrowing gap with generalist foundation models suggests that continued data and model scaling can deliver domain-relevant gains and position them as strong foundations for future medical foundation models.
Is an Ultra Large Natural Image-Based Foundation Model Superior to a Retina-Specific Model for Detecting Ocular and Systemic Diseases?
Feb 10, 2025Abstract:The advent of foundation models (FMs) is transforming medical domain. In ophthalmology, RETFound, a retina-specific FM pre-trained sequentially on 1.4 million natural images and 1.6 million retinal images, has demonstrated high adaptability across clinical applications. Conversely, DINOv2, a general-purpose vision FM pre-trained on 142 million natural images, has shown promise in non-medical domains. However, its applicability to clinical tasks remains underexplored. To address this, we conducted head-to-head evaluations by fine-tuning RETFound and three DINOv2 models (large, base, small) for ocular disease detection and systemic disease prediction tasks, across eight standardized open-source ocular datasets, as well as the Moorfields AlzEye and the UK Biobank datasets. DINOv2-large model outperformed RETFound in detecting diabetic retinopathy (AUROC=0.850-0.952 vs 0.823-0.944, across three datasets, all P<=0.007) and multi-class eye diseases (AUROC=0.892 vs. 0.846, P<0.001). In glaucoma, DINOv2-base model outperformed RETFound (AUROC=0.958 vs 0.940, P<0.001). Conversely, RETFound achieved superior performance over all DINOv2 models in predicting heart failure, myocardial infarction, and ischaemic stroke (AUROC=0.732-0.796 vs 0.663-0.771, all P<0.001). These trends persisted even with 10% of the fine-tuning data. These findings showcase the distinct scenarios where general-purpose and domain-specific FMs excel, highlighting the importance of aligning FM selection with task-specific requirements to optimise clinical performance.
VAFO-Loss: VAscular Feature Optimised Loss Function for Retinal Artery/Vein Segmentation
Mar 12, 2022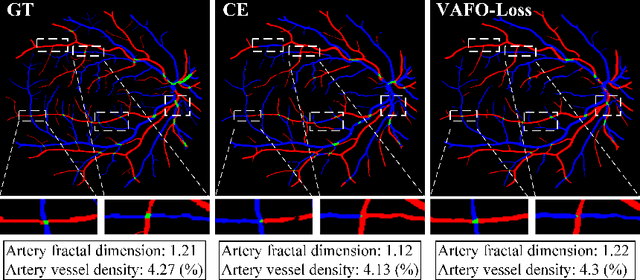
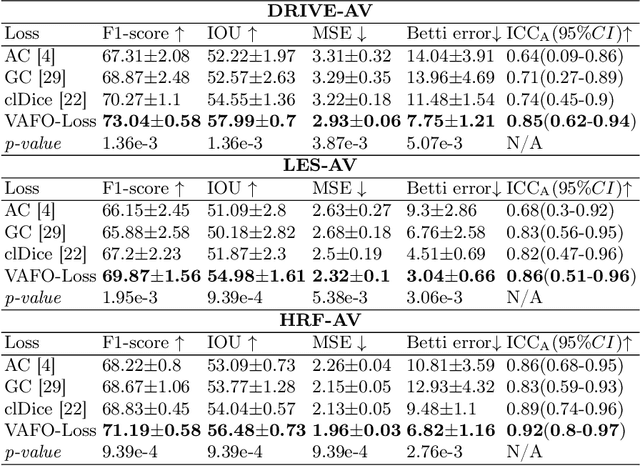
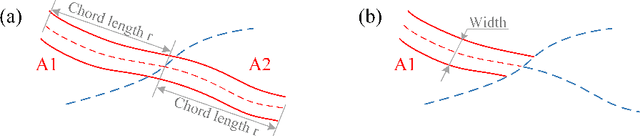
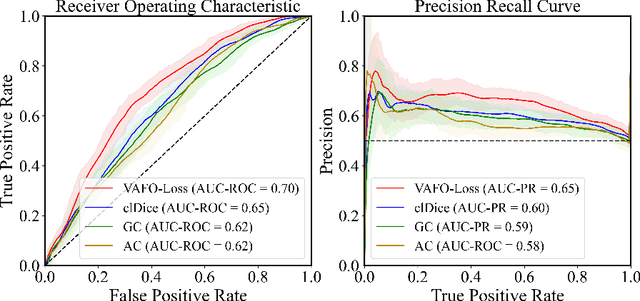
Abstract:Estimating clinically-relevant vascular features following vessel segmentation is a standard pipeline for retinal vessel analysis, which provides potential ocular biomarkers for both ophthalmic disease and systemic disease. In this work, we integrate these clinical features into a novel vascular feature optimised loss function (VAFO-Loss), in order to regularise networks to produce segmentation maps, with which more accurate vascular features can be derived. Two common vascular features, vessel density and fractal dimension, are identified to be sensitive to intra-segment misclassification, which is a well-recognised problem in multi-class artery/vein segmentation particularly hindering the estimation of these vascular features. Thus we encode these two features into VAFO-Loss. We first show that incorporating our end-to-end VAFO-Loss in standard segmentation networks indeed improves vascular feature estimation, yielding quantitative improvement in stroke incidence prediction, a clinical downstream task. We also report a technically interesting finding that the trained segmentation network, albeit biased by the feature optimised loss VAFO-Loss, shows statistically significant improvement in segmentation metrics, compared to those trained with other state-of-the-art segmentation losses.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge