Michael A. Riegler
FANet: A Feedback Attention Network for Improved Biomedical Image Segmentation
Mar 31, 2021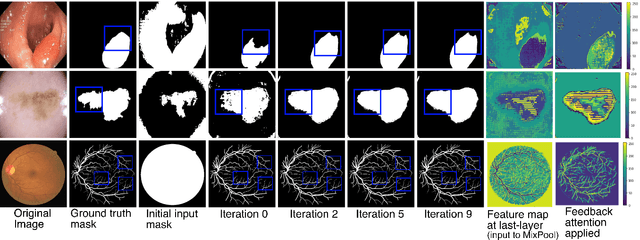
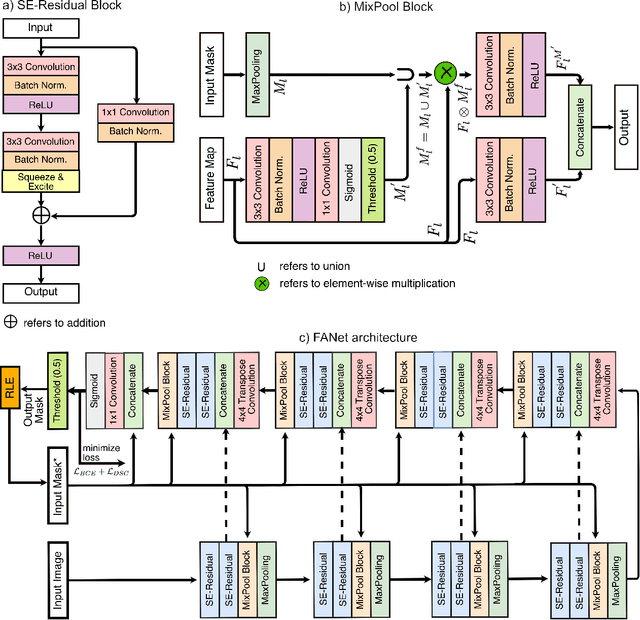
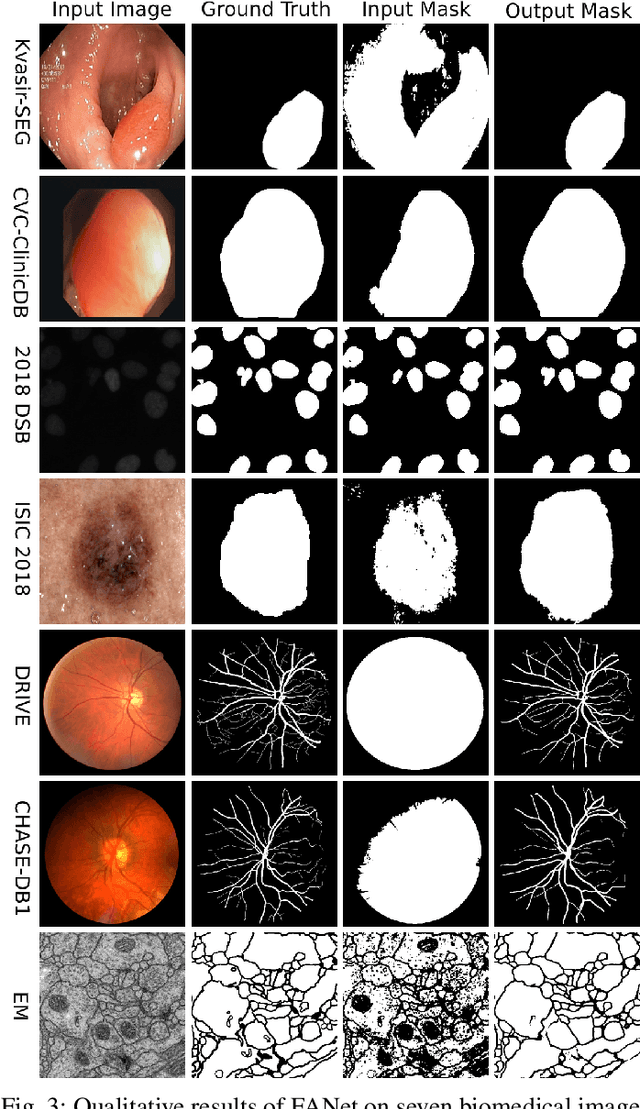
Abstract:With the increase in available large clinical and experimental datasets, there has been substantial amount of work being done on addressing the challenges in the area of biomedical image analysis. Image segmentation, which is crucial for any quantitative analysis, has especially attracted attention. Recent hardware advancement has led to the success of deep learning approaches. However, although deep learning models are being trained on large datasets, existing methods do not use the information from different learning epochs effectively. In this work, we leverage the information of each training epoch to prune the prediction maps of the subsequent epochs. We propose a novel architecture called feedback attention network (FANet) that unifies the previous epoch mask with the feature map of the current training epoch. The previous epoch mask is then used to provide a hard attention to the learnt feature maps at different convolutional layers. The network also allows to rectify the predictions in an iterative fashion during the test time. We show that our proposed feedback attention model provides a substantial improvement on most segmentation metrics tested on seven publicly available biomedical imaging datasets demonstrating the effectiveness of the proposed FANet.
LightLayers: Parameter Efficient Dense and Convolutional Layers for Image Classification
Jan 06, 2021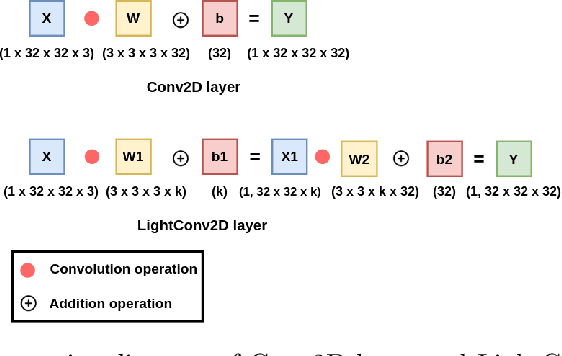
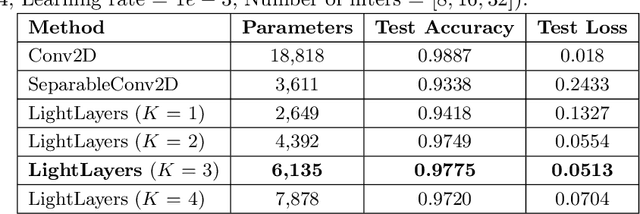
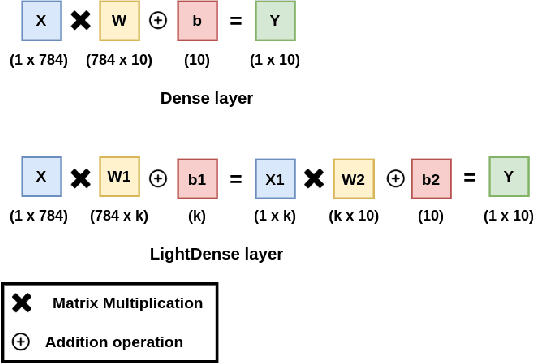
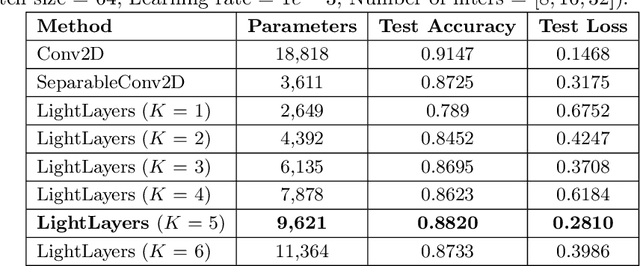
Abstract:Deep Neural Networks (DNNs) have become the de-facto standard in computer vision, as well as in many other pattern recognition tasks. A key drawback of DNNs is that the training phase can be very computationally expensive. Organizations or individuals that cannot afford purchasing state-of-the-art hardware or tapping into cloud-hosted infrastructures may face a long waiting time before the training completes or might not be able to train a model at all. Investigating novel ways to reduce the training time could be a potential solution to alleviate this drawback, and thus enabling more rapid development of new algorithms and models. In this paper, we propose LightLayers, a method for reducing the number of trainable parameters in deep neural networks (DNN). The proposed LightLayers consists of LightDense andLightConv2D layer that are as efficient as regular Conv2D and Dense layers, but uses less parameters. We resort to Matrix Factorization to reduce the complexity of the DNN models resulting into lightweight DNNmodels that require less computational power, without much loss in the accuracy. We have tested LightLayers on MNIST, Fashion MNIST, CI-FAR 10, and CIFAR 100 datasets. Promising results are obtained for MNIST, Fashion MNIST, CIFAR-10 datasets whereas CIFAR 100 shows acceptable performance by using fewer parameters.
DDANet: Dual Decoder Attention Network for Automatic Polyp Segmentation
Dec 30, 2020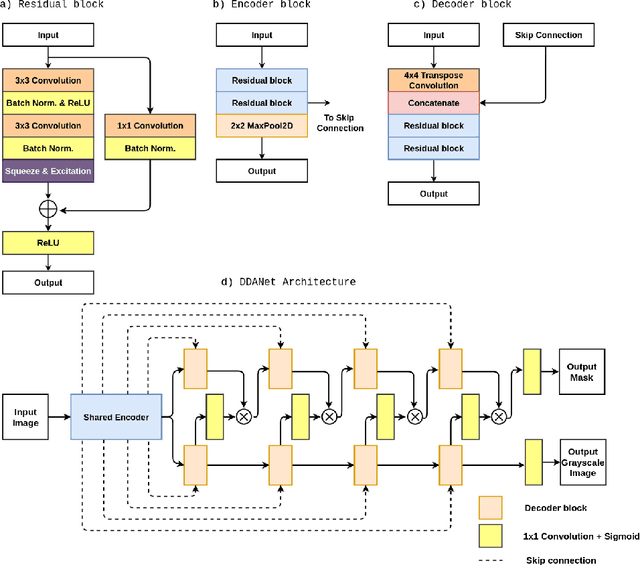
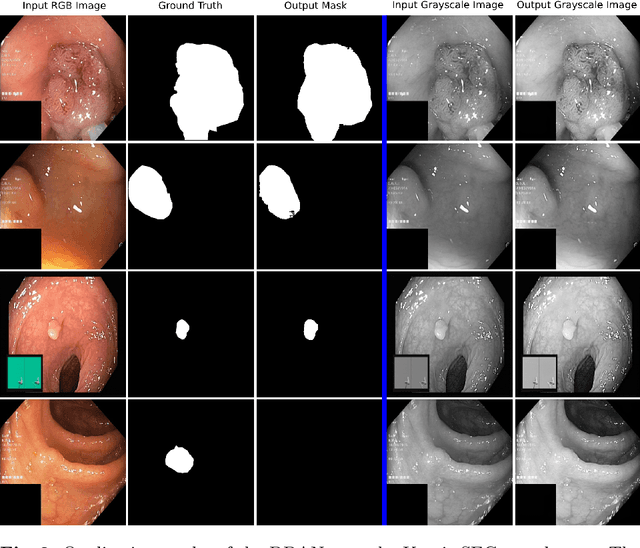
Abstract:Colonoscopy is the gold standard for examination and detection of colorectal polyps. Localization and delineation of polyps can play a vital role in treatment (e.g., surgical planning) and prognostic decision making. Polyp segmentation can provide detailed boundary information for clinical analysis. Convolutional neural networks have improved the performance in colonoscopy. However, polyps usually possess various challenges, such as intra-and inter-class variation and noise. While manual labeling for polyp assessment requires time from experts and is prone to human error (e.g., missed lesions), an automated, accurate, and fast segmentation can improve the quality of delineated lesion boundaries and reduce missed rate. The Endotect challenge provides an opportunity to benchmark computer vision methods by training on the publicly available Hyperkvasir and testing on a separate unseen dataset. In this paper, we propose a novel architecture called ``DDANet'' based on a dual decoder attention network. Our experiments demonstrate that the model trained on the Kvasir-SEG dataset and tested on an unseen dataset achieves a dice coefficient of 0.7874, mIoU of 0.7010, recall of 0.7987, and a precision of 0.8577, demonstrating the generalization ability of our model.
Medico Multimedia Task at MediaEval 2020: Automatic Polyp Segmentation
Dec 30, 2020

Abstract:Colorectal cancer is the third most common cause of cancer worldwide. According to Global cancer statistics 2018, the incidence of colorectal cancer is increasing in both developing and developed countries. Early detection of colon anomalies such as polyps is important for cancer prevention, and automatic polyp segmentation can play a crucial role for this. Regardless of the recent advancement in early detection and treatment options, the estimated polyp miss rate is still around 20\%. Support via an automated computer-aided diagnosis system could be one of the potential solutions for the overlooked polyps. Such detection systems can help low-cost design solutions and save doctors time, which they could for example use to perform more patient examinations. In this paper, we introduce the 2020 Medico challenge, provide some information on related work and the dataset, describe the task and evaluation metrics, and discuss the necessity of organizing the Medico challenge.
Pyramid-Focus-Augmentation: Medical Image Segmentation with Step-Wise Focus
Dec 14, 2020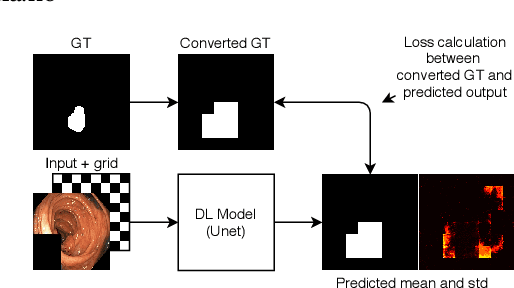
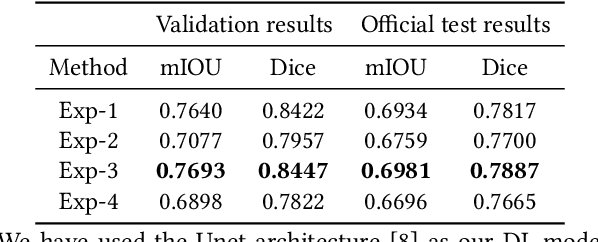
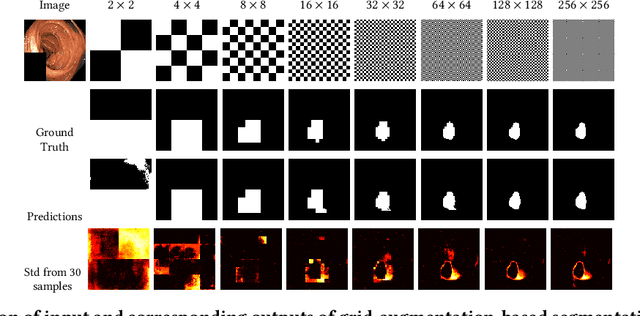
Abstract:Segmentation of findings in the gastrointestinal tract is a challenging but also an important task which is an important building stone for sufficient automatic decision support systems. In this work, we present our solution for the Medico 2020 task, which focused on the problem of colon polyp segmentation. We present our simple but efficient idea of using an augmentation method that uses grids in a pyramid-like manner (large to small) for segmentation. Our results show that the proposed methods work as indented and can also lead to comparable results when competing with other methods.
Real-Time Polyp Detection, Localisation and Segmentation in Colonoscopy Using Deep Learning
Nov 15, 2020
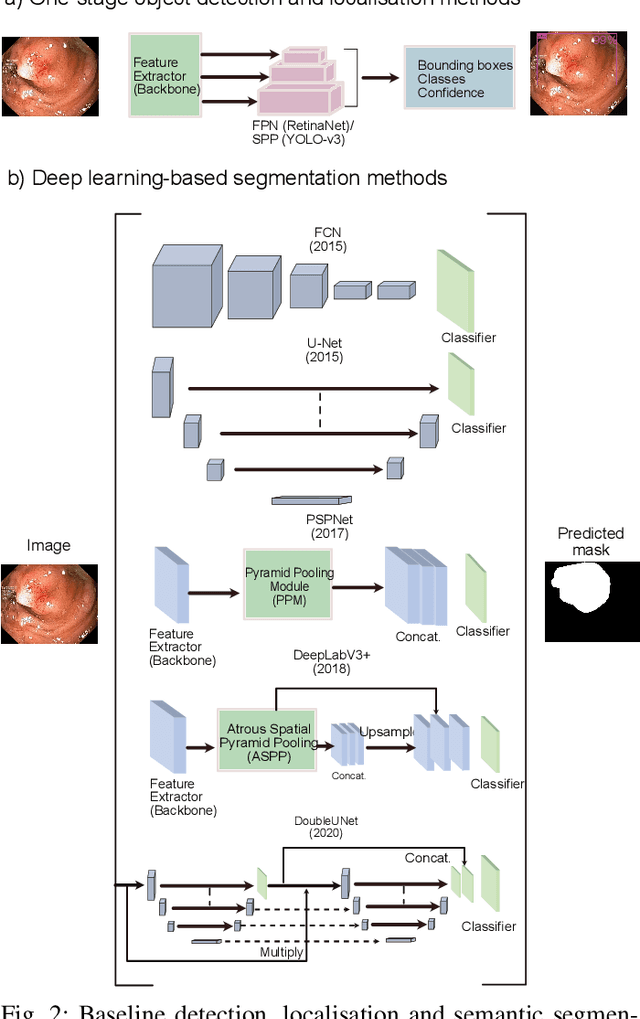
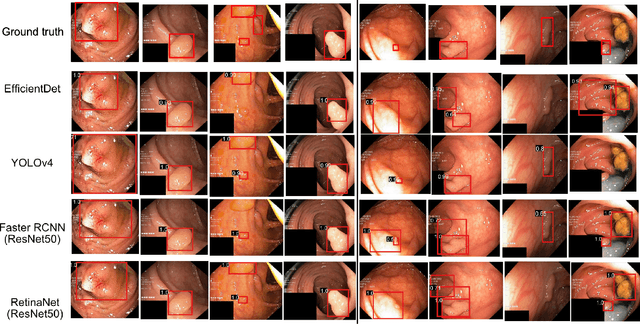
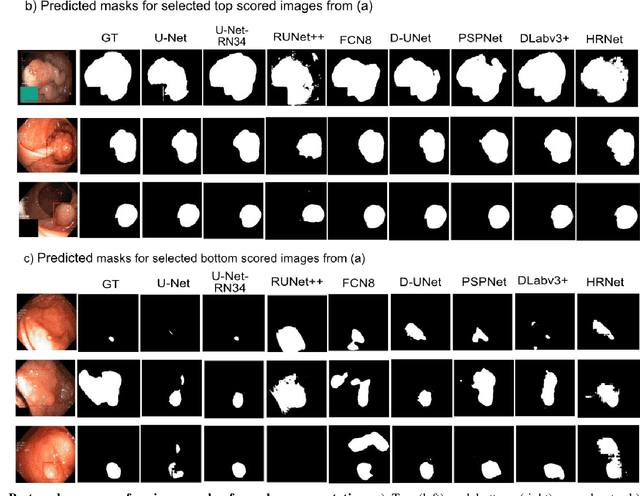
Abstract:Computer-aided detection, localisation, and segmentation methods can help improve colonoscopy procedures. Even though many methods have been built to tackle automatic detection and segmentation of polyps, benchmarking of state-of-the-art methods still remains an open problem. This is due to the increasing number of researched computer-vision methods that can be applied to polyp datasets. Benchmarking of novel methods can provide a direction to the development of automated polyp detection and segmentation tasks. Furthermore, it ensures that the produced results in the community are reproducible and provide a fair comparison of developed methods. In this paper, we benchmark several recent state-of-the-art methods using Kvasir-SEG, an open-access dataset of colonoscopy images, for polyp detection, localisation, and segmentation evaluating both method accuracy and speed. Whilst, most methods in literature have competitive performance over accuracy, we show that YOLOv4 with a Darknet53 backbone and cross-stage-partial connections achieved a better trade-off between an average precision of 0.8513 and mean IoU of 0.8025, and the fastest speed of 48 frames per second for the detection and localisation task. Likewise, UNet with a ResNet34 backbone achieved the highest dice coefficient of 0.8757 and the best average speed of 35 frames per second for the segmentation task. Our comprehensive comparison with various state-of-the-art methods reveal the importance of benchmarking the deep learning methods for automated real-time polyp identification and delineations that can potentially transform current clinical practices and minimise miss-detection rates.
DoubleU-Net: A Deep Convolutional Neural Network for Medical Image Segmentation
Jun 27, 2020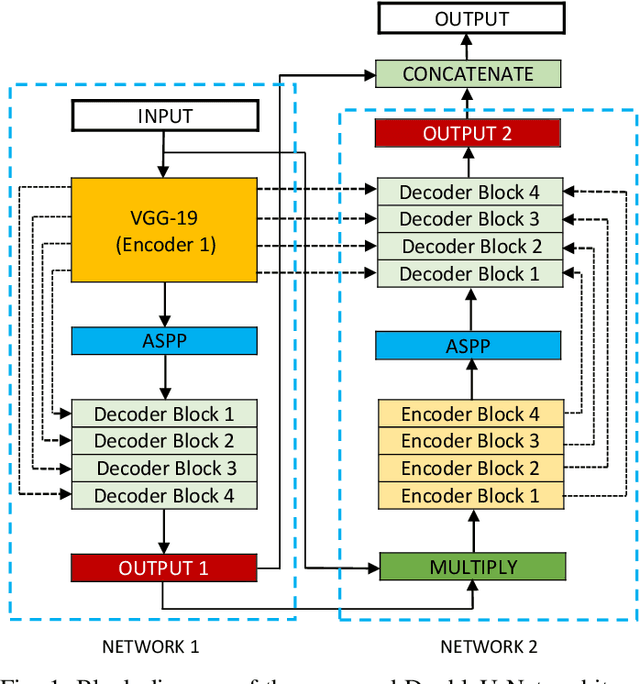
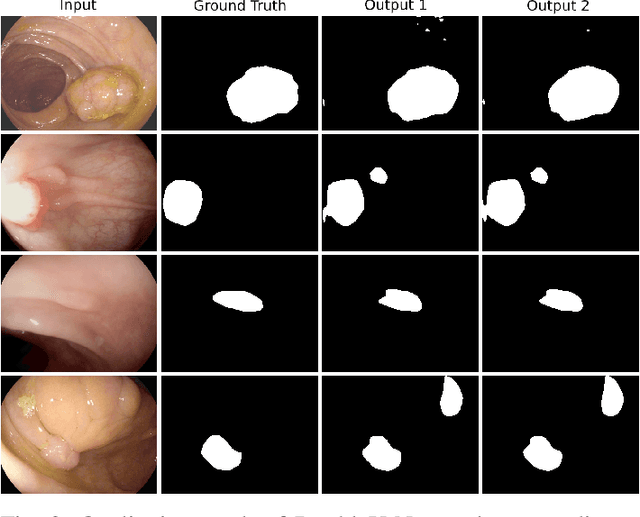
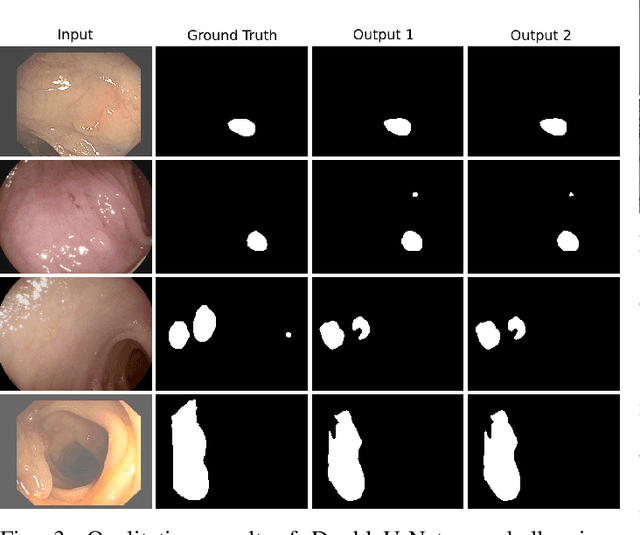
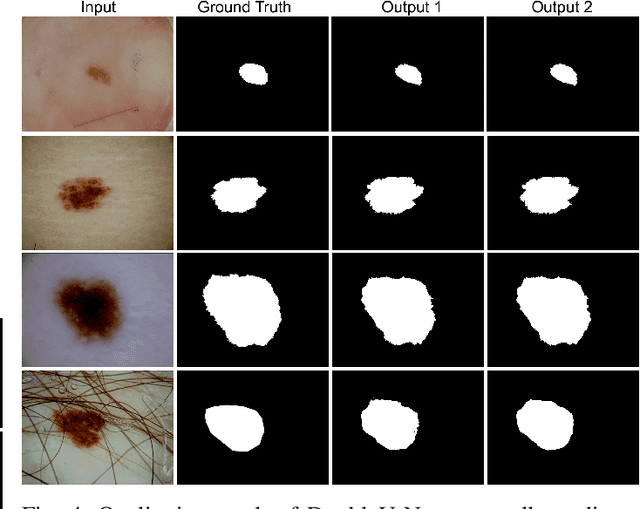
Abstract:Semantic image segmentation is the process of labeling each pixel of an image with its corresponding class. An encoder-decoder based approach, like U-Net and its variants, is a popular strategy for solving medical image segmentation tasks. To improve the performance of U-Net on various segmentation tasks, we propose a novel architecture called DoubleU-Net, which is a combination of two U-Net architectures stacked on top of each other. The first U-Net uses a pre-trained VGG-19 as the encoder, which has already learned features from ImageNet and can be transferred to another task easily. To capture more semantic information efficiently, we added another U-Net at the bottom. We also adopt Atrous Spatial Pyramid Pooling (ASPP) to capture contextual information within the network. We have evaluated DoubleU-Net using four medical segmentation datasets, covering various imaging modalities such as colonoscopy, dermoscopy, and microscopy. Experiments on the MICCAI 2015 segmentation challenge, the CVC-ClinicDB, the 2018 Data Science Bowl challenge, and the Lesion boundary segmentation datasets demonstrate that the DoubleU-Net outperforms U-Net and the baseline models. Moreover, DoubleU-Net produces more accurate segmentation masks, especially in the case of the CVC-ClinicDB and MICCAI 2015 segmentation challenge datasets, which have challenging images such as smaller and flat polyps. These results show the improvement over the existing U-Net model. The encouraging results, produced on various medical image segmentation datasets, show that DoubleU-Net can be used as a strong baseline for both medical image segmentation and cross-dataset evaluation testing to measure the generalizability of Deep Learning (DL) models.
An Extensive Study on Cross-Dataset Bias and Evaluation Metrics Interpretation for Machine Learning applied to Gastrointestinal Tract Abnormality Classification
May 08, 2020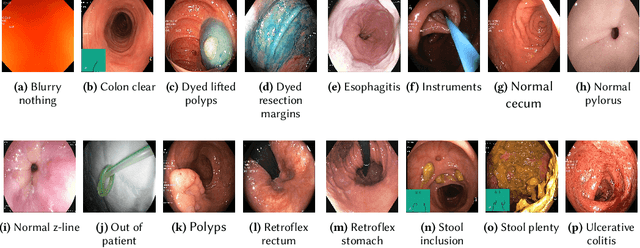
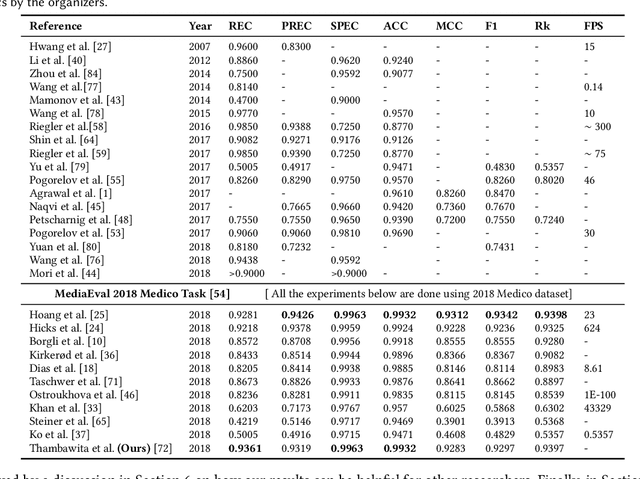
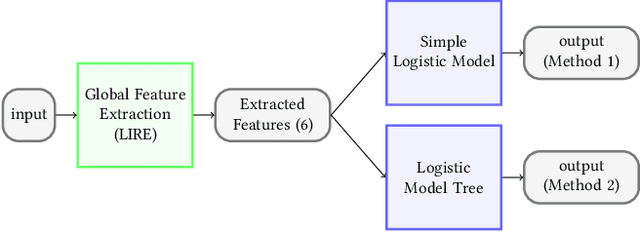
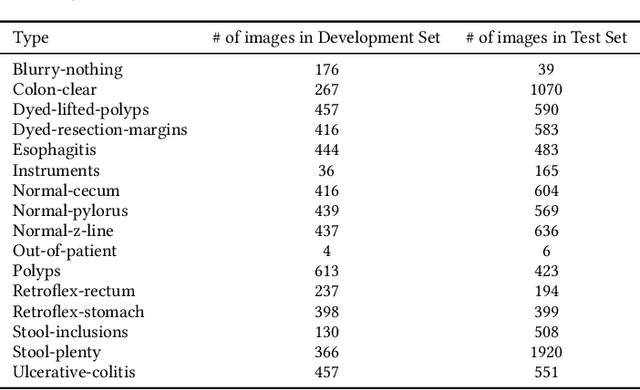
Abstract:Precise and efficient automated identification of Gastrointestinal (GI) tract diseases can help doctors treat more patients and improve the rate of disease detection and identification. Currently, automatic analysis of diseases in the GI tract is a hot topic in both computer science and medical-related journals. Nevertheless, the evaluation of such an automatic analysis is often incomplete or simply wrong. Algorithms are often only tested on small and biased datasets, and cross-dataset evaluations are rarely performed. A clear understanding of evaluation metrics and machine learning models with cross datasets is crucial to bring research in the field to a new quality level. Towards this goal, we present comprehensive evaluations of five distinct machine learning models using Global Features and Deep Neural Networks that can classify 16 different key types of GI tract conditions, including pathological findings, anatomical landmarks, polyp removal conditions, and normal findings from images captured by common GI tract examination instruments. In our evaluation, we introduce performance hexagons using six performance metrics such as recall, precision, specificity, accuracy, F1-score, and Matthews Correlation Coefficient to demonstrate how to determine the real capabilities of models rather than evaluating them shallowly. Furthermore, we perform cross-dataset evaluations using different datasets for training and testing. With these cross-dataset evaluations, we demonstrate the challenge of actually building a generalizable model that could be used across different hospitals. Our experiments clearly show that more sophisticated performance metrics and evaluation methods need to be applied to get reliable models rather than depending on evaluations of the splits of the same dataset, i.e., the performance metrics should always be interpreted together rather than relying on a single metric.
Efficient Quantile Tracking Using an Oracle
Apr 27, 2020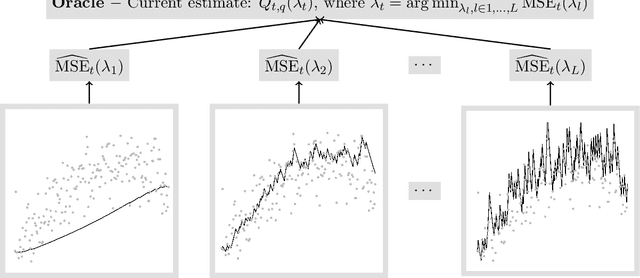
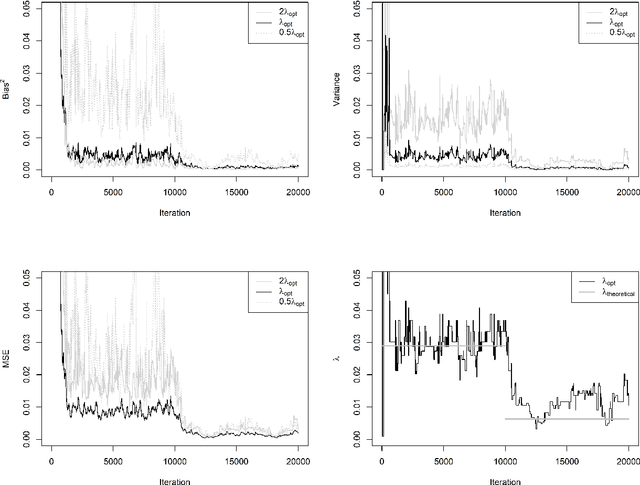
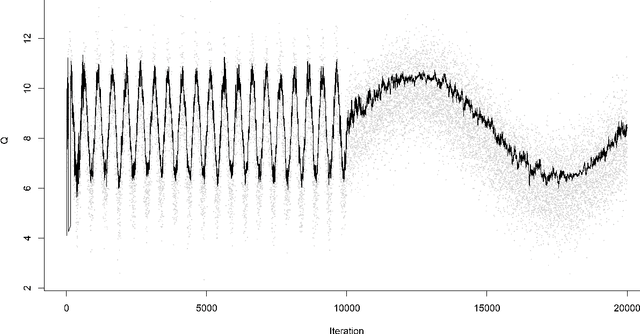
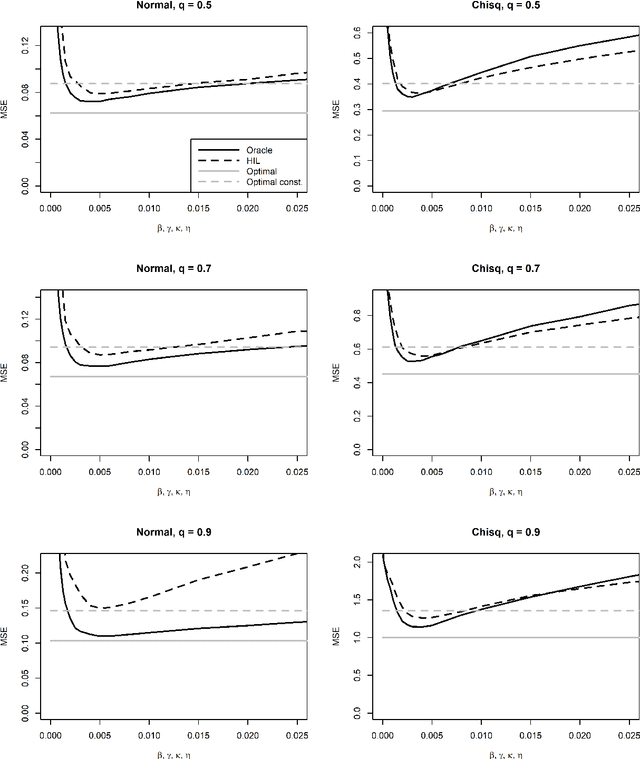
Abstract:For incremental quantile estimators the step size and possibly other tuning parameters must be carefully set. However, little attention has been given on how to set these values in an online manner. In this article we suggest two novel procedures that address this issue. The core part of the procedures is to estimate the current tracking mean squared error (MSE). The MSE is decomposed in tracking variance and bias and novel and efficient procedures to estimate these quantities are presented. It is shown that estimation bias can be tracked by associating it with the portion of observations below the quantile estimates. The first procedure runs an ensemble of $L$ quantile estimators for wide range of values of the tuning parameters and typically around $L = 100$. In each iteration an oracle selects the best estimate by the guidance of the estimated MSEs. The second method only runs an ensemble of $L = 3$ estimators and thus the values of the tuning parameters need from time to time to be adjusted for the running estimators. The procedures have a low memory foot print of $8L$ and a computational complexity of $8L$ per iteration. The experiments show that the procedures are highly efficient and track quantiles with an error close to the theoretical optimum. The Oracle approach performs best, but comes with higher computational cost. The procedures were further applied to a massive real-life data stream of tweets and proofed real world applicability of them.
Robust Medical Instrument Segmentation Challenge 2019
Mar 23, 2020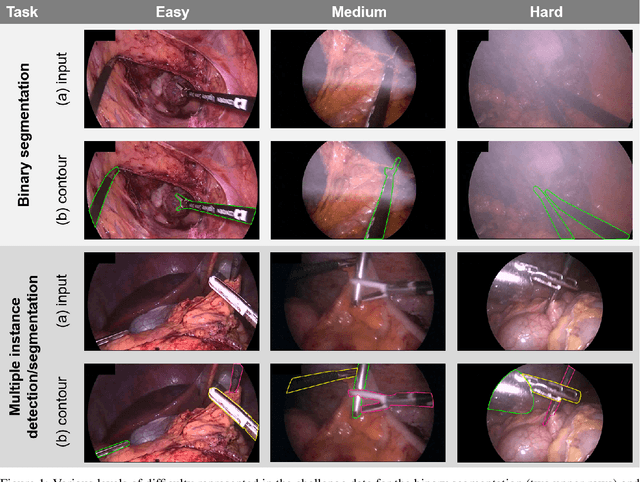
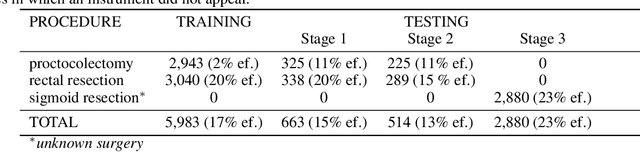
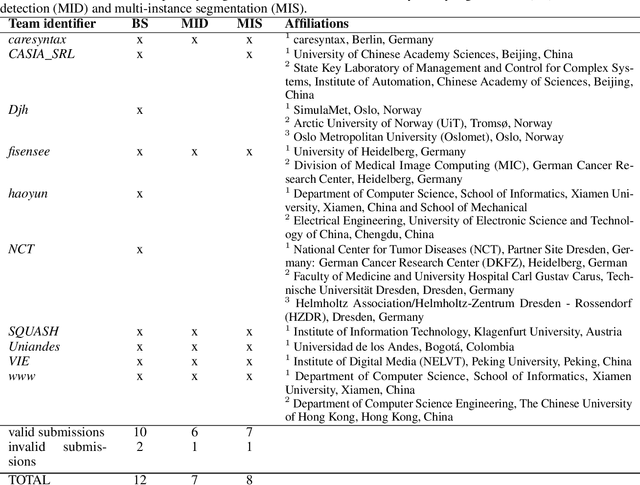
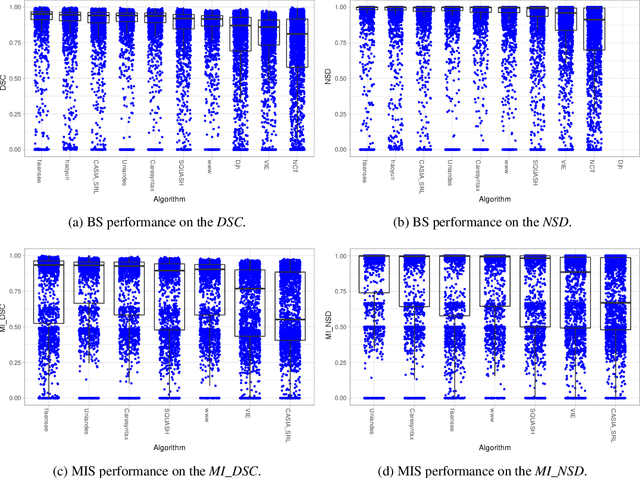
Abstract:Intraoperative tracking of laparoscopic instruments is often a prerequisite for computer and robotic-assisted interventions. While numerous methods for detecting, segmenting and tracking of medical instruments based on endoscopic video images have been proposed in the literature, key limitations remain to be addressed: Firstly, robustness, that is, the reliable performance of state-of-the-art methods when run on challenging images (e.g. in the presence of blood, smoke or motion artifacts). Secondly, generalization; algorithms trained for a specific intervention in a specific hospital should generalize to other interventions or institutions. In an effort to promote solutions for these limitations, we organized the Robust Medical Instrument Segmentation (ROBUST-MIS) challenge as an international benchmarking competition with a specific focus on the robustness and generalization capabilities of algorithms. For the first time in the field of endoscopic image processing, our challenge included a task on binary segmentation and also addressed multi-instance detection and segmentation. The challenge was based on a surgical data set comprising 10,040 annotated images acquired from a total of 30 surgical procedures from three different types of surgery. The validation of the competing methods for the three tasks (binary segmentation, multi-instance detection and multi-instance segmentation) was performed in three different stages with an increasing domain gap between the training and the test data. The results confirm the initial hypothesis, namely that algorithm performance degrades with an increasing domain gap. While the average detection and segmentation quality of the best-performing algorithms is high, future research should concentrate on detection and segmentation of small, crossing, moving and transparent instrument(s) (parts).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge