Ischemic Stroke Lesion Segmentation
Ischemic stroke lesion segmentation is the process of identifying and segmenting lesions in brain MRI scans for medical diagnosis.
Papers and Code
ISLA: A U-Net for MRI-based acute ischemic stroke lesion segmentation with deep supervision, attention, domain adaptation, and ensemble learning
Jan 13, 2026Accurate delineation of acute ischemic stroke lesions in MRI is a key component of stroke diagnosis and management. In recent years, deep learning models have been successfully applied to the automatic segmentation of such lesions. While most proposed architectures are based on the U-Net framework, they primarily differ in their choice of loss functions and in the use of deep supervision, residual connections, and attention mechanisms. Moreover, many implementations are not publicly available, and the optimal configuration for acute ischemic stroke (AIS) lesion segmentation remains unclear. In this work, we introduce ISLA (Ischemic Stroke Lesion Analyzer), a new deep learning model for AIS lesion segmentation from diffusion MRI, trained on three multicenter databases totaling more than 1500 AIS participants. Through systematic optimization of the loss function, convolutional architecture, deep supervision, and attention mechanisms, we developed a robust segmentation framework. We further investigated unsupervised domain adaptation to improve generalization to an external clinical dataset. ISLA outperformed two state-of-the-art approaches for AIS lesion segmentation on an external test set. Codes and trained models will be made publicly available to facilitate reuse and reproducibility.
Dual-Encoder Transformer-Based Multimodal Learning for Ischemic Stroke Lesion Segmentation Using Diffusion MRI
Dec 23, 2025Accurate segmentation of ischemic stroke lesions from diffusion magnetic resonance imaging (MRI) is essential for clinical decision-making and outcome assessment. Diffusion-Weighted Imaging (DWI) and Apparent Diffusion Coefficient (ADC) scans provide complementary information on acute and sub-acute ischemic changes; however, automated lesion delineation remains challenging due to variability in lesion appearance. In this work, we study ischemic stroke lesion segmentation using multimodal diffusion MRI from the ISLES 2022 dataset. Several state-of-the-art convolutional and transformer-based architectures, including U-Net variants, Swin-UNet, and TransUNet, are benchmarked. Based on performance, a dual-encoder TransUNet architecture is proposed to learn modality-specific representations from DWI and ADC inputs. To incorporate spatial context, adjacent slice information is integrated using a three-slice input configuration. All models are trained under a unified framework and evaluated using the Dice Similarity Coefficient (DSC). Results show that transformer-based models outperform convolutional baselines, and the proposed dual-encoder TransUNet achieves the best performance, reaching a Dice score of 85.4% on the test set. The proposed framework offers a robust solution for automated ischemic stroke lesion segmentation from diffusion MRI.
An Explainable Agentic AI Framework for Uncertainty-Aware and Abstention-Enabled Acute Ischemic Stroke Imaging Decisions
Jan 03, 2026Artificial intelligence models have shown strong potential in acute ischemic stroke imaging, particularly for lesion detection and segmentation using computed tomography and magnetic resonance imaging. However, most existing approaches operate as black box predictors, producing deterministic outputs without explicit uncertainty awareness or structured mechanisms to abstain under ambiguous conditions. This limitation raises serious safety and trust concerns in high risk emergency radiology settings. In this paper, we propose an explainable agentic AI framework for uncertainty aware and abstention enabled decision support in acute ischemic stroke imaging. The framework follows a modular agentic pipeline in which a perception agent performs lesion aware image analysis, an uncertainty estimation agent computes slice level predictive reliability, and a decision agent determines whether to issue a prediction or abstain based on predefined uncertainty thresholds. Unlike prior stroke imaging systems that primarily focus on improving segmentation or classification accuracy, the proposed framework explicitly prioritizes clinical safety, transparency, and clinician aligned decision behavior. Qualitative and case based analyses across representative stroke imaging scenarios demonstrate that uncertainty driven abstention naturally emerges in diagnostically ambiguous regions and low information slices. The framework further integrates visual explanation mechanisms to support both predictive and abstention decisions, addressing a key limitation of existing uncertainty aware medical imaging systems. Rather than introducing a new performance benchmark, this work presents agentic control, uncertainty awareness, and selective abstention as essential design principles for developing safe and trustworthy medical imaging AI systems.
Segmentation of Ischemic Stroke Lesions using Transfer Learning on Multi-sequence MRI
Nov 10, 2025The accurate understanding of ischemic stroke lesions is critical for efficient therapy and prognosis of stroke patients. Magnetic resonance imaging (MRI) is sensitive to acute ischemic stroke and is a common diagnostic method for stroke. However, manual lesion segmentation performed by experts is tedious, time-consuming, and prone to observer inconsistency. Automatic medical image analysis methods have been proposed to overcome this challenge. However, previous approaches have relied on hand-crafted features that may not capture the irregular and physiologically complex shapes of ischemic stroke lesions. In this study, we present a novel framework for quickly and automatically segmenting ischemic stroke lesions on various MRI sequences, including T1-weighted, T2-weighted, DWI, and FLAIR. The proposed methodology is validated on the ISLES 2015 Brain Stroke sequence dataset, where we trained our model using the Res-Unet architecture twice: first, with pre-existing weights, and then without, to explore the benefits of transfer learning. Evaluation metrics, including the Dice score and sensitivity, were computed across 3D volumes. Finally, a Majority Voting Classifier was integrated to amalgamate the outcomes from each axis, resulting in a comprehensive segmentation method. Our efforts culminated in achieving a Dice score of 80.5\% and an accuracy of 74.03\%, showcasing the efficacy of our segmentation approach.
AREPAS: Anomaly Detection in Fine-Grained Anatomy with Reconstruction-Based Semantic Patch-Scoring
Sep 16, 2025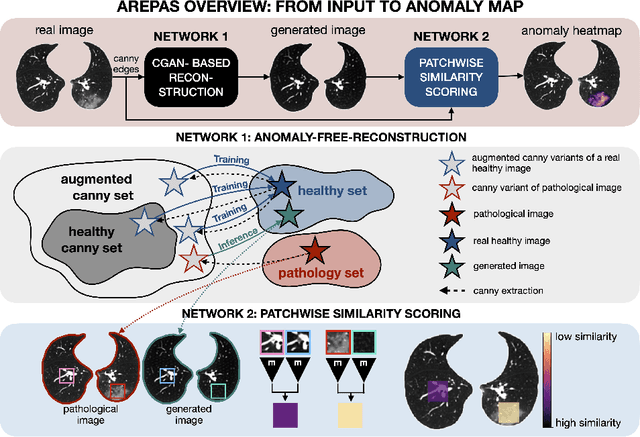
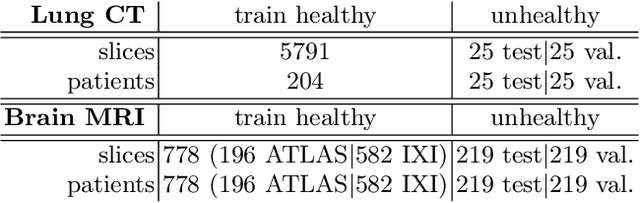
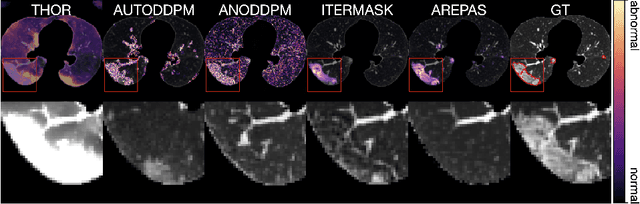
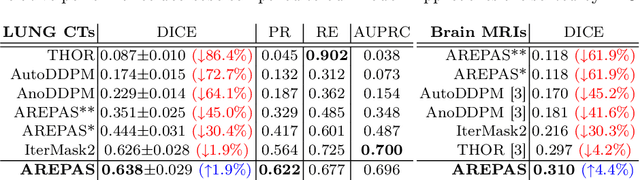
Early detection of newly emerging diseases, lesion severity assessment, differentiation of medical conditions and automated screening are examples for the wide applicability and importance of anomaly detection (AD) and unsupervised segmentation in medicine. Normal fine-grained tissue variability such as present in pulmonary anatomy is a major challenge for existing generative AD methods. Here, we propose a novel generative AD approach addressing this issue. It consists of an image-to-image translation for anomaly-free reconstruction and a subsequent patch similarity scoring between observed and generated image-pairs for precise anomaly localization. We validate the new method on chest computed tomography (CT) scans for the detection and segmentation of infectious disease lesions. To assess generalizability, we evaluate the method on an ischemic stroke lesion segmentation task in T1-weighted brain MRI. Results show improved pixel-level anomaly segmentation in both chest CTs and brain MRIs, with relative DICE score improvements of +1.9% and +4.4%, respectively, compared to other state-of-the-art reconstruction-based methods.
Deep Learning-Driven Segmentation of Ischemic Stroke Lesions Using Multi-Channel MRI
Jan 04, 2025



Ischemic stroke, caused by cerebral vessel occlusion, presents substantial challenges in medical imaging due to the variability and subtlety of stroke lesions. Magnetic Resonance Imaging (MRI) plays a crucial role in diagnosing and managing ischemic stroke, yet existing segmentation techniques often fail to accurately delineate lesions. This study introduces a novel deep learning-based method for segmenting ischemic stroke lesions using multi-channel MRI modalities, including Diffusion Weighted Imaging (DWI), Apparent Diffusion Coefficient (ADC), and enhanced Diffusion Weighted Imaging (eDWI). The proposed architecture integrates DenseNet121 as the encoder with Self-Organized Operational Neural Networks (SelfONN) in the decoder, enhanced by Channel and Space Compound Attention (CSCA) and Double Squeeze-and-Excitation (DSE) blocks. Additionally, a custom loss function combining Dice Loss and Jaccard Loss with weighted averages is introduced to improve model performance. Trained and evaluated on the ISLES 2022 dataset, the model achieved Dice Similarity Coefficients (DSC) of 83.88% using DWI alone, 85.86% with DWI and ADC, and 87.49% with the integration of DWI, ADC, and eDWI. This approach not only outperforms existing methods but also addresses key limitations in current segmentation practices. These advancements significantly enhance diagnostic precision and treatment planning for ischemic stroke, providing valuable support for clinical decision-making.
Generalizable automated ischaemic stroke lesion segmentation with vision transformers
Feb 10, 2025



Ischaemic stroke, a leading cause of death and disability, critically relies on neuroimaging for characterising the anatomical pattern of injury. Diffusion-weighted imaging (DWI) provides the highest expressivity in ischemic stroke but poses substantial challenges for automated lesion segmentation: susceptibility artefacts, morphological heterogeneity, age-related comorbidities, time-dependent signal dynamics, instrumental variability, and limited labelled data. Current U-Net-based models therefore underperform, a problem accentuated by inadequate evaluation metrics that focus on mean performance, neglecting anatomical, subpopulation, and acquisition-dependent variability. Here, we present a high-performance DWI lesion segmentation tool addressing these challenges through optimized vision transformer-based architectures, integration of 3563 annotated lesions from multi-site data, and algorithmic enhancements, achieving state-of-the-art results. We further propose a novel evaluative framework assessing model fidelity, equity (across demographics and lesion subtypes), anatomical precision, and robustness to instrumental variability, promoting clinical and research utility. This work advances stroke imaging by reconciling model expressivity with domain-specific challenges and redefining performance benchmarks to prioritize equity and generalizability, critical for personalized medicine and mechanistic research.
Automated Segmentation of Ischemic Stroke Lesions in Non-Contrast Computed Tomography Images for Enhanced Treatment and Prognosis
Nov 14, 2024Stroke is the second leading cause of death worldwide, and is increasingly prevalent in low- and middle-income countries (LMICs). Timely interventions can significantly influence stroke survivability and the quality of life after treatment. However, the standard and most widely available imaging method for confirming strokes and their sub-types, the NCCT, is more challenging and time-consuming to employ in cases of ischemic stroke. For this reason, we developed an automated method for ischemic stroke lesion segmentation in NCCTs using the nnU-Net frame work, aimed at enhancing early treatment and improving the prognosis of ischemic stroke patients. We achieved Dice scores of 0.596 and Intersection over Union (IoU) scores of 0.501 on the sampled dataset. After adjusting for outliers, these scores improved to 0.752 for the Dice score and 0.643 for the IoU. Proper delineation of the region of infarction can help clinicians better assess the potential impact of the infarction, and guide treatment procedures.
ISLES 2024: The first longitudinal multimodal multi-center real-world dataset in (sub-)acute stroke
Aug 20, 2024
Stroke remains a leading cause of global morbidity and mortality, placing a heavy socioeconomic burden. Over the past decade, advances in endovascular reperfusion therapy and the use of CT and MRI imaging for treatment guidance have significantly improved patient outcomes and are now standard in clinical practice. To develop machine learning algorithms that can extract meaningful and reproducible models of brain function for both clinical and research purposes from stroke images - particularly for lesion identification, brain health quantification, and prognosis - large, diverse, and well-annotated public datasets are essential. While only a few datasets with (sub-)acute stroke data were previously available, several large, high-quality datasets have recently been made publicly accessible. However, these existing datasets include only MRI data. In contrast, our dataset is the first to offer comprehensive longitudinal stroke data, including acute CT imaging with angiography and perfusion, follow-up MRI at 2-9 days, as well as acute and longitudinal clinical data up to a three-month outcome. The dataset includes a training dataset of n = 150 and a test dataset of n = 100 scans. Training data is publicly available, while test data will be used exclusively for model validation. We are making this dataset available as part of the 2024 edition of the Ischemic Stroke Lesion Segmentation (ISLES) challenge (https://www.isles-challenge.org/), which continuously aims to establish benchmark methods for acute and sub-acute ischemic stroke lesion segmentation, aiding in creating open stroke imaging datasets and evaluating cutting-edge image processing algorithms.
A Robust Ensemble Algorithm for Ischemic Stroke Lesion Segmentation: Generalizability and Clinical Utility Beyond the ISLES Challenge
Apr 03, 2024



Diffusion-weighted MRI (DWI) is essential for stroke diagnosis, treatment decisions, and prognosis. However, image and disease variability hinder the development of generalizable AI algorithms with clinical value. We address this gap by presenting a novel ensemble algorithm derived from the 2022 Ischemic Stroke Lesion Segmentation (ISLES) challenge. ISLES'22 provided 400 patient scans with ischemic stroke from various medical centers, facilitating the development of a wide range of cutting-edge segmentation algorithms by the research community. Through collaboration with leading teams, we combined top-performing algorithms into an ensemble model that overcomes the limitations of individual solutions. Our ensemble model achieved superior ischemic lesion detection and segmentation accuracy on our internal test set compared to individual algorithms. This accuracy generalized well across diverse image and disease variables. Furthermore, the model excelled in extracting clinical biomarkers. Notably, in a Turing-like test, neuroradiologists consistently preferred the algorithm's segmentations over manual expert efforts, highlighting increased comprehensiveness and precision. Validation using a real-world external dataset (N=1686) confirmed the model's generalizability. The algorithm's outputs also demonstrated strong correlations with clinical scores (admission NIHSS and 90-day mRS) on par with or exceeding expert-derived results, underlining its clinical relevance. This study offers two key findings. First, we present an ensemble algorithm (https://github.com/Tabrisrei/ISLES22_Ensemble) that detects and segments ischemic stroke lesions on DWI across diverse scenarios on par with expert (neuro)radiologists. Second, we show the potential for biomedical challenge outputs to extend beyond the challenge's initial objectives, demonstrating their real-world clinical applicability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge