Yiran Jiang
Feature-Space Planes Searcher: A Universal Domain Adaptation Framework for Interpretability and Computational Efficiency
Aug 26, 2025Abstract:Domain shift, characterized by degraded model performance during transition from labeled source domains to unlabeled target domains, poses a persistent challenge for deploying deep learning systems. Current unsupervised domain adaptation (UDA) methods predominantly rely on fine-tuning feature extractors - an approach limited by inefficiency, reduced interpretability, and poor scalability to modern architectures. Our analysis reveals that models pretrained on large-scale data exhibit domain-invariant geometric patterns in their feature space, characterized by intra-class clustering and inter-class separation, thereby preserving transferable discriminative structures. These findings indicate that domain shifts primarily manifest as boundary misalignment rather than feature degradation. Unlike fine-tuning entire pre-trained models - which risks introducing unpredictable feature distortions - we propose the Feature-space Planes Searcher (FPS): a novel domain adaptation framework that optimizes decision boundaries by leveraging these geometric patterns while keeping the feature encoder frozen. This streamlined approach enables interpretative analysis of adaptation while substantially reducing memory and computational costs through offline feature extraction, permitting full-dataset optimization in a single computation cycle. Evaluations on public benchmarks demonstrate that FPS achieves competitive or superior performance to state-of-the-art methods. FPS scales efficiently with multimodal large models and shows versatility across diverse domains including protein structure prediction, remote sensing classification, and earthquake detection. We anticipate FPS will provide a simple, effective, and generalizable paradigm for transfer learning, particularly in domain adaptation tasks. .
Clinical Assistant Diagnosis for Electronic Medical Record Based on Convolutional Neural Network
Apr 23, 2018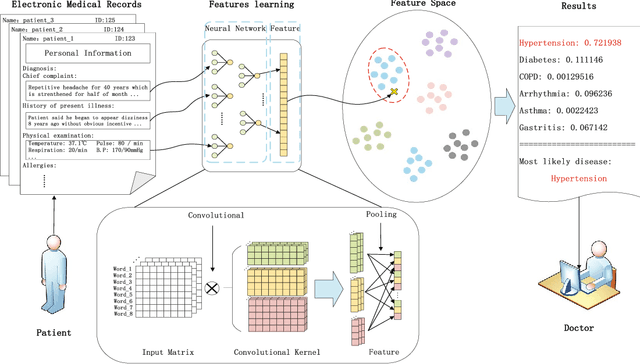
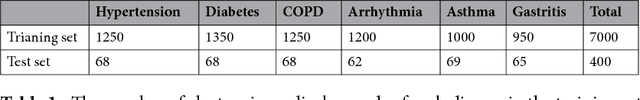
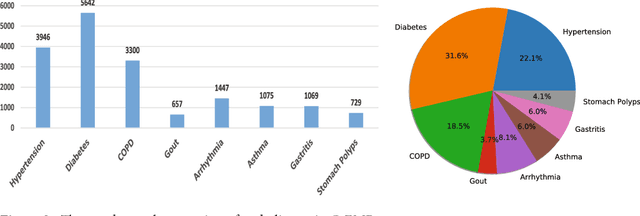
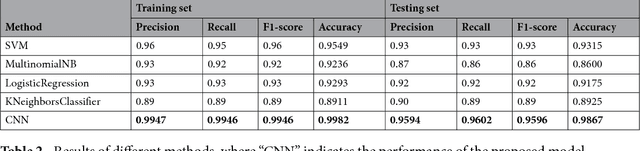
Abstract:Automatically extracting useful information from electronic medical records along with conducting disease diagnoses is a promising task for both clinical decision support(CDS) and neural language processing(NLP). Most of the existing systems are based on artificially constructed knowledge bases, and then auxiliary diagnosis is done by rule matching. In this study, we present a clinical intelligent decision approach based on Convolutional Neural Networks(CNN), which can automatically extract high-level semantic information of electronic medical records and then perform automatic diagnosis without artificial construction of rules or knowledge bases. We use collected 18,590 copies of the real-world clinical electronic medical records to train and test the proposed model. Experimental results show that the proposed model can achieve 98.67\% accuracy and 96.02\% recall, which strongly supports that using convolutional neural network to automatically learn high-level semantic features of electronic medical records and then conduct assist diagnosis is feasible and effective.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge