Gabor Fichtinger
A Dataset and Benchmarks for Atrial Fibrillation Detection from Electrocardiograms of Intensive Care Unit Patients
Dec 19, 2025Abstract:Objective: Atrial fibrillation (AF) is the most common cardiac arrhythmia experienced by intensive care unit (ICU) patients and can cause adverse health effects. In this study, we publish a labelled ICU dataset and benchmarks for AF detection. Methods: We compared machine learning models across three data-driven artificial intelligence (AI) approaches: feature-based classifiers, deep learning (DL), and ECG foundation models (FMs). This comparison addresses a critical gap in the literature and aims to pinpoint which AI approach is best for accurate AF detection. Electrocardiograms (ECGs) from a Canadian ICU and the 2021 PhysioNet/Computing in Cardiology Challenge were used to conduct the experiments. Multiple training configurations were tested, ranging from zero-shot inference to transfer learning. Results: On average and across both datasets, ECG FMs performed best, followed by DL, then feature-based classifiers. The model that achieved the top F1 score on our ICU test set was ECG-FM through a transfer learning strategy (F1=0.89). Conclusion: This study demonstrates promising potential for using AI to build an automatic patient monitoring system. Significance: By publishing our labelled ICU dataset (LinkToBeAdded) and performance benchmarks, this work enables the research community to continue advancing the state-of-the-art in AF detection in the ICU.
FACT: Foundation Model for Assessing Cancer Tissue Margins with Mass Spectrometry
Apr 15, 2025Abstract:Purpose: Accurately classifying tissue margins during cancer surgeries is crucial for ensuring complete tumor removal. Rapid Evaporative Ionization Mass Spectrometry (REIMS), a tool for real-time intraoperative margin assessment, generates spectra that require machine learning models to support clinical decision-making. However, the scarcity of labeled data in surgical contexts presents a significant challenge. This study is the first to develop a foundation model tailored specifically for REIMS data, addressing this limitation and advancing real-time surgical margin assessment. Methods: We propose FACT, a Foundation model for Assessing Cancer Tissue margins. FACT is an adaptation of a foundation model originally designed for text-audio association, pretrained using our proposed supervised contrastive approach based on triplet loss. An ablation study is performed to compare our proposed model against other models and pretraining methods. Results: Our proposed model significantly improves the classification performance, achieving state-of-the-art performance with an AUROC of $82.4\% \pm 0.8$. The results demonstrate the advantage of our proposed pretraining method and selected backbone over the self-supervised and semi-supervised baselines and alternative models. Conclusion: Our findings demonstrate that foundation models, adapted and pretrained using our novel approach, can effectively classify REIMS data even with limited labeled examples. This highlights the viability of foundation models for enhancing real-time surgical margin assessment, particularly in data-scarce clinical environments.
Surgical Data Science -- from Concepts to Clinical Translation
Oct 30, 2020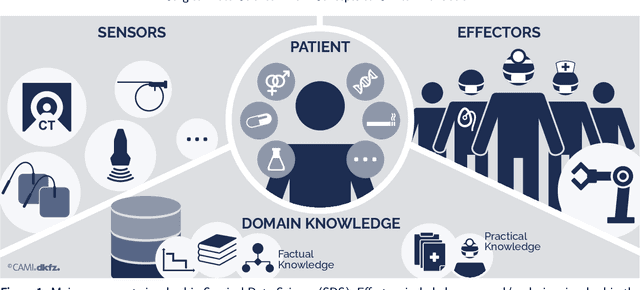
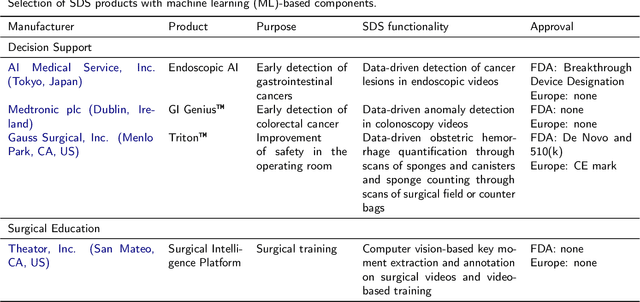
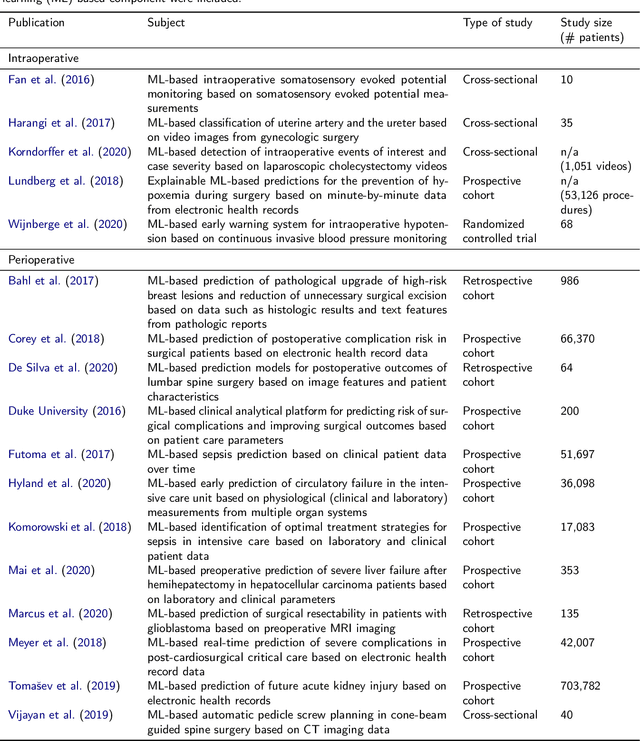
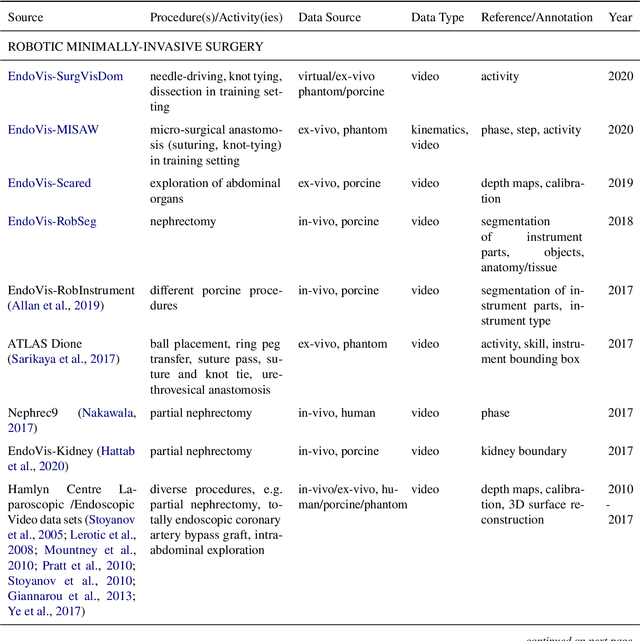
Abstract:Recent developments in data science in general and machine learning in particular have transformed the way experts envision the future of surgery. Surgical data science is a new research field that aims to improve the quality of interventional healthcare through the capture, organization, analysis and modeling of data. While an increasing number of data-driven approaches and clinical applications have been studied in the fields of radiological and clinical data science, translational success stories are still lacking in surgery. In this publication, we shed light on the underlying reasons and provide a roadmap for future advances in the field. Based on an international workshop involving leading researchers in the field of surgical data science, we review current practice, key achievements and initiatives as well as available standards and tools for a number of topics relevant to the field, namely (1) technical infrastructure for data acquisition, storage and access in the presence of regulatory constraints, (2) data annotation and sharing and (3) data analytics. Drawing from this extensive review, we present current challenges for technology development and (4) describe a roadmap for faster clinical translation and exploitation of the full potential of surgical data science.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge