Andy Kim
Alignment Pretraining: AI Discourse Causes Self-Fulfilling (Mis)alignment
Jan 15, 2026Abstract:Pretraining corpora contain extensive discourse about AI systems, yet the causal influence of this discourse on downstream alignment remains poorly understood. If prevailing descriptions of AI behaviour are predominantly negative, LLMs may internalise corresponding behavioural priors, giving rise to self-fulfilling misalignment. This paper provides the first controlled study of this hypothesis by pretraining 6.9B-parameter LLMs with varying amounts of (mis)alignment discourse. We find that discussion of AI contributes to misalignment. Upsampling synthetic training documents about AI misalignment leads to a notable increase in misaligned behaviour. Conversely, upsampling documents about aligned behaviour reduces misalignment scores from 45% to 9%. We consider this evidence of self-fulfilling alignment. These effects are dampened, but persist through post-training. Our findings establish the study of how pretraining data shapes alignment priors, or alignment pretraining, as a complement to post-training. We recommend practitioners pretrain for alignment as well as capabilities. Our models and datasets are available at alignmentpretraining.ai
CheXbreak: Misclassification Identification for Deep Learning Models Interpreting Chest X-rays
Mar 24, 2021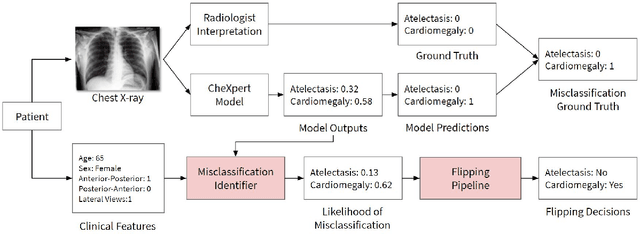
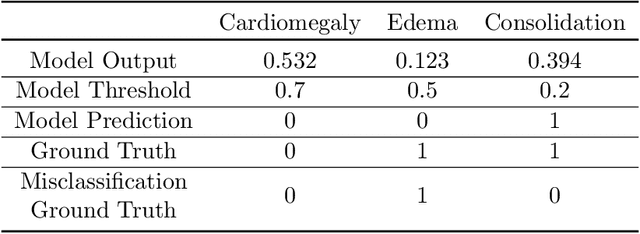
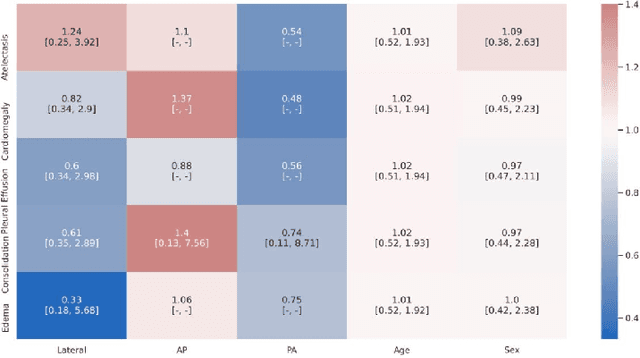
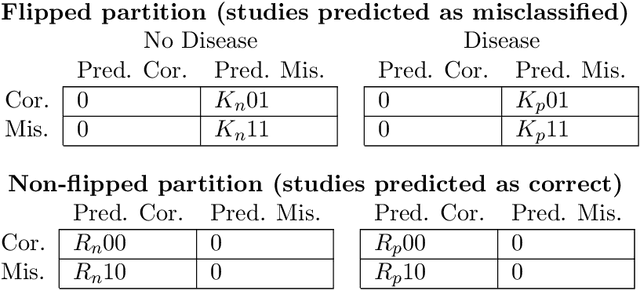
Abstract:A major obstacle to the integration of deep learning models for chest x-ray interpretation into clinical settings is the lack of understanding of their failure modes. In this work, we first investigate whether there are patient subgroups that chest x-ray models are likely to misclassify. We find that patient age and the radiographic finding of lung lesion, pneumothorax or support devices are statistically relevant features for predicting misclassification for some chest x-ray models. Second, we develop misclassification predictors on chest x-ray models using their outputs and clinical features. We find that our best performing misclassification identifier achieves an AUROC close to 0.9 for most diseases. Third, employing our misclassification identifiers, we develop a corrective algorithm to selectively flip model predictions that have high likelihood of misclassification at inference time. We observe F1 improvement on the prediction of Consolidation (0.008 [95\% CI 0.005, 0.010]) and Edema (0.003, [95\% CI 0.001, 0.006]). By carrying out our investigation on ten distinct and high-performing chest x-ray models, we are able to derive insights across model architectures and offer a generalizable framework applicable to other medical imaging tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge