Adrian Galdran
Understanding metric-related pitfalls in image analysis validation
Feb 09, 2023Abstract:Validation metrics are key for the reliable tracking of scientific progress and for bridging the current chasm between artificial intelligence (AI) research and its translation into practice. However, increasing evidence shows that particularly in image analysis, metrics are often chosen inadequately in relation to the underlying research problem. This could be attributed to a lack of accessibility of metric-related knowledge: While taking into account the individual strengths, weaknesses, and limitations of validation metrics is a critical prerequisite to making educated choices, the relevant knowledge is currently scattered and poorly accessible to individual researchers. Based on a multi-stage Delphi process conducted by a multidisciplinary expert consortium as well as extensive community feedback, the present work provides the first reliable and comprehensive common point of access to information on pitfalls related to validation metrics in image analysis. Focusing on biomedical image analysis but with the potential of transfer to other fields, the addressed pitfalls generalize across application domains and are categorized according to a newly created, domain-agnostic taxonomy. To facilitate comprehension, illustrations and specific examples accompany each pitfall. As a structured body of information accessible to researchers of all levels of expertise, this work enhances global comprehension of a key topic in image analysis validation.
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Class Adaptive Network Calibration
Nov 28, 2022



Abstract:Recent studies have revealed that, beyond conventional accuracy, calibration should also be considered for training modern deep neural networks. To address miscalibration during learning, some methods have explored different penalty functions as part of the learning objective, alongside a standard classification loss, with a hyper-parameter controlling the relative contribution of each term. Nevertheless, these methods share two major drawbacks: 1) the scalar balancing weight is the same for all classes, hindering the ability to address different intrinsic difficulties or imbalance among classes; and 2) the balancing weight is usually fixed without an adaptive strategy, which may prevent from reaching the best compromise between accuracy and calibration, and requires hyper-parameter search for each application. We propose Class Adaptive Label Smoothing (CALS) for calibrating deep networks, which allows to learn class-wise multipliers during training, yielding a powerful alternative to common label smoothing penalties. Our method builds on a general Augmented Lagrangian approach, a well-established technique in constrained optimization, but we introduce several modifications to tailor it for large-scale, class-adaptive training. Comprehensive evaluation and multiple comparisons on a variety of benchmarks, including standard and long-tailed image classification, semantic segmentation, and text classification, demonstrate the superiority of the proposed method. The code is available at https://github.com/by-liu/CALS.
On the Optimal Combination of Cross-Entropy and Soft Dice Losses for Lesion Segmentation with Out-of-Distribution Robustness
Sep 14, 2022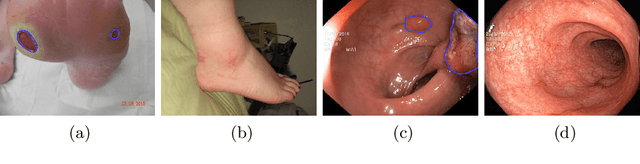
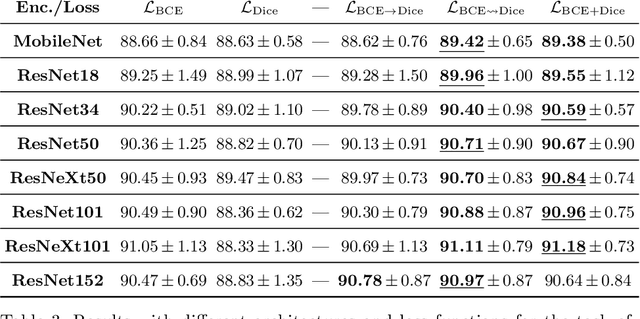
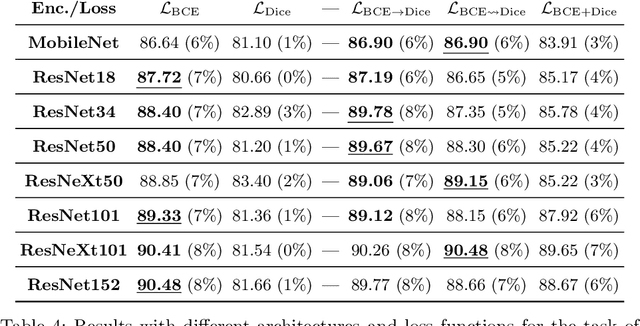
Abstract:We study the impact of different loss functions on lesion segmentation from medical images. Although the Cross-Entropy (CE) loss is the most popular option when dealing with natural images, for biomedical image segmentation the soft Dice loss is often preferred due to its ability to handle imbalanced scenarios. On the other hand, the combination of both functions has also been successfully applied in this kind of tasks. A much less studied problem is the generalization ability of all these losses in the presence of Out-of-Distribution (OoD) data. This refers to samples appearing in test time that are drawn from a different distribution than training images. In our case, we train our models on images that always contain lesions, but in test time we also have lesion-free samples. We analyze the impact of the minimization of different loss functions on in-distribution performance, but also its ability to generalize to OoD data, via comprehensive experiments on polyp segmentation from endoscopic images and ulcer segmentation from diabetic feet images. Our findings are surprising: CE-Dice loss combinations that excel in segmenting in-distribution images have a poor performance when dealing with OoD data, which leads us to recommend the adoption of the CE loss for this kind of problems, due to its robustness and ability to generalize to OoD samples. Code associated to our experiments can be found at https://github.com/agaldran/lesion_losses_ood .
Calibrating Segmentation Networks with Margin-based Label Smoothing
Sep 09, 2022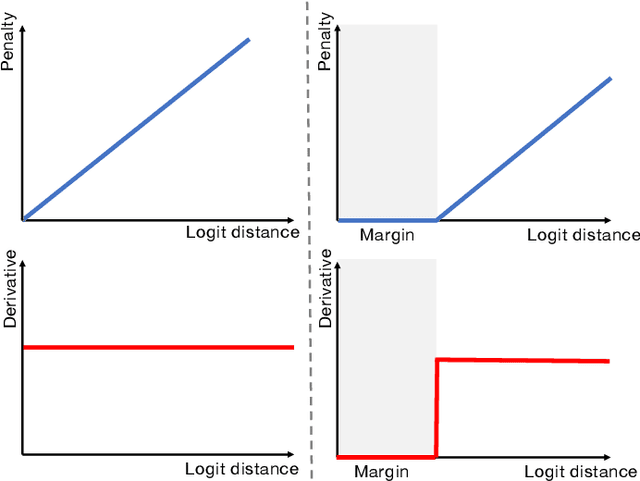
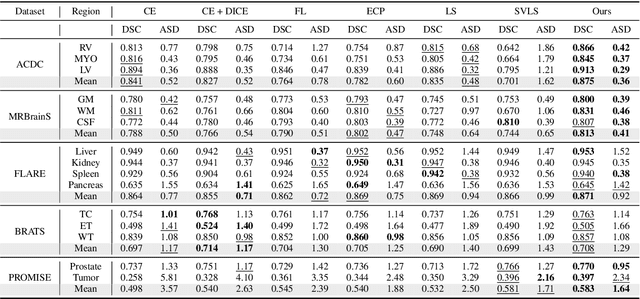
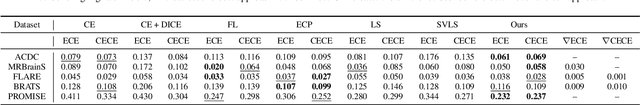
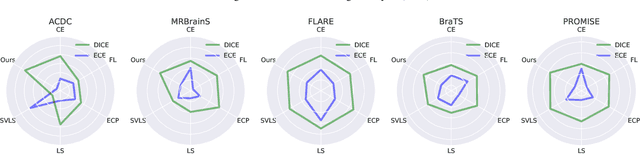
Abstract:Despite the undeniable progress in visual recognition tasks fueled by deep neural networks, there exists recent evidence showing that these models are poorly calibrated, resulting in over-confident predictions. The standard practices of minimizing the cross entropy loss during training promote the predicted softmax probabilities to match the one-hot label assignments. Nevertheless, this yields a pre-softmax activation of the correct class that is significantly larger than the remaining activations, which exacerbates the miscalibration problem. Recent observations from the classification literature suggest that loss functions that embed implicit or explicit maximization of the entropy of predictions yield state-of-the-art calibration performances. Despite these findings, the impact of these losses in the relevant task of calibrating medical image segmentation networks remains unexplored. In this work, we provide a unifying constrained-optimization perspective of current state-of-the-art calibration losses. Specifically, these losses could be viewed as approximations of a linear penalty (or a Lagrangian term) imposing equality constraints on logit distances. This points to an important limitation of such underlying equality constraints, whose ensuing gradients constantly push towards a non-informative solution, which might prevent from reaching the best compromise between the discriminative performance and calibration of the model during gradient-based optimization. Following our observations, we propose a simple and flexible generalization based on inequality constraints, which imposes a controllable margin on logit distances. Comprehensive experiments on a variety of public medical image segmentation benchmarks demonstrate that our method sets novel state-of-the-art results on these tasks in terms of network calibration, whereas the discriminative performance is also improved.
Test Time Transform Prediction for Open Set Histopathological Image Recognition
Jun 27, 2022
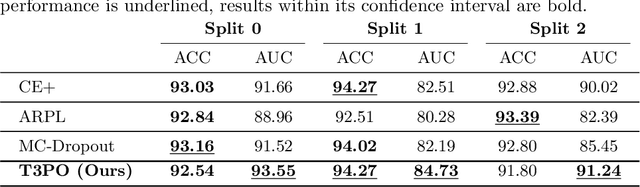
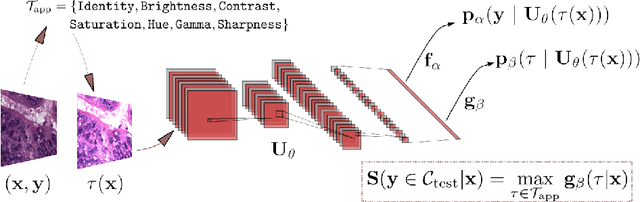
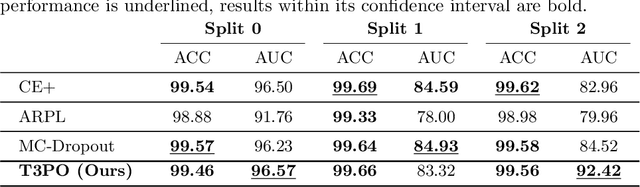
Abstract:Tissue typology annotation in Whole Slide histological images is a complex and tedious, yet necessary task for the development of computational pathology models. We propose to address this problem by applying Open Set Recognition techniques to the task of jointly classifying tissue that belongs to a set of annotated classes, e.g. clinically relevant tissue categories, while rejecting in test time Open Set samples, i.e. images that belong to categories not present in the training set. To this end, we introduce a new approach for Open Set histopathological image recognition based on training a model to accurately identify image categories and simultaneously predict which data augmentation transform has been applied. In test time, we measure model confidence in predicting this transform, which we expect to be lower for images in the Open Set. We carry out comprehensive experiments in the context of colorectal cancer assessment from histological images, which provide evidence on the strengths of our approach to automatically identify samples from unknown categories. Code is released at https://github.com/agaldran/t3po .
Assessing generalisability of deep learning-based polyp detection and segmentation methods through a computer vision challenge
Feb 24, 2022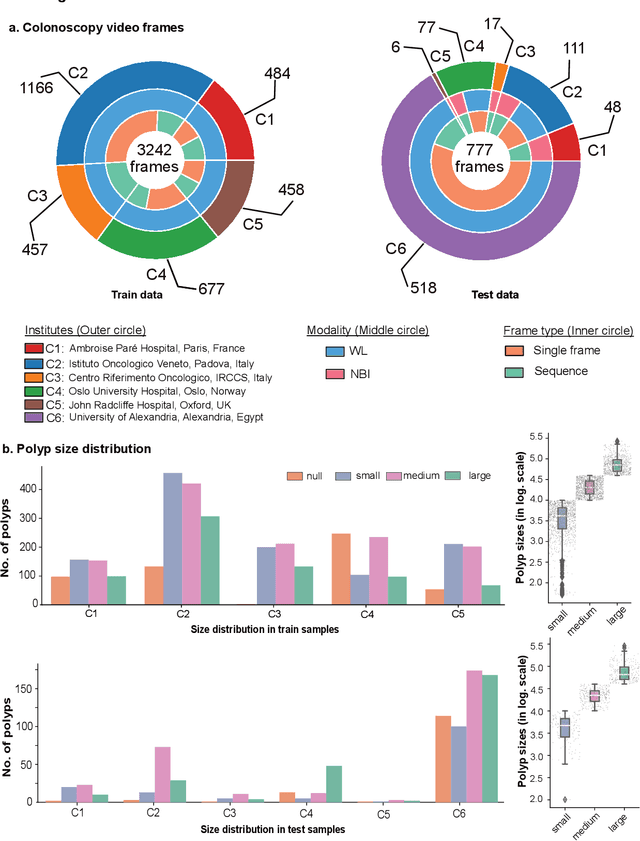
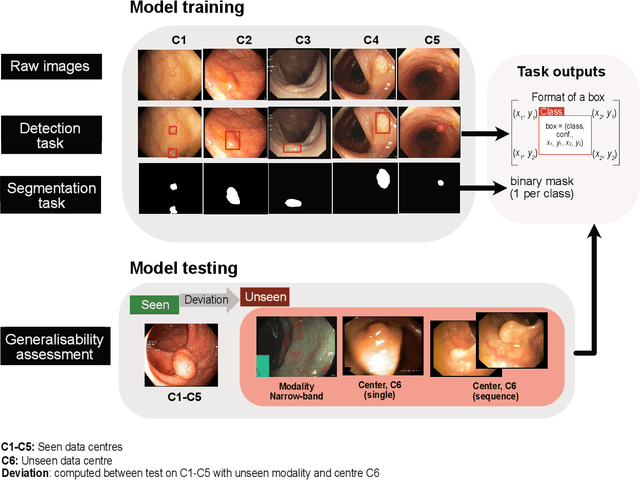
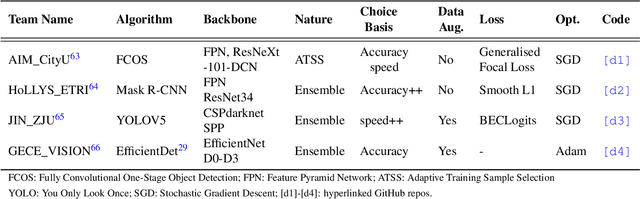
Abstract:Polyps are well-known cancer precursors identified by colonoscopy. However, variability in their size, location, and surface largely affect identification, localisation, and characterisation. Moreover, colonoscopic surveillance and removal of polyps (referred to as polypectomy ) are highly operator-dependent procedures. There exist a high missed detection rate and incomplete removal of colonic polyps due to their variable nature, the difficulties to delineate the abnormality, the high recurrence rates, and the anatomical topography of the colon. There have been several developments in realising automated methods for both detection and segmentation of these polyps using machine learning. However, the major drawback in most of these methods is their ability to generalise to out-of-sample unseen datasets that come from different centres, modalities and acquisition systems. To test this hypothesis rigorously we curated a multi-centre and multi-population dataset acquired from multiple colonoscopy systems and challenged teams comprising machine learning experts to develop robust automated detection and segmentation methods as part of our crowd-sourcing Endoscopic computer vision challenge (EndoCV) 2021. In this paper, we analyse the detection results of the four top (among seven) teams and the segmentation results of the five top teams (among 16). Our analyses demonstrate that the top-ranking teams concentrated on accuracy (i.e., accuracy > 80% on overall Dice score on different validation sets) over real-time performance required for clinical applicability. We further dissect the methods and provide an experiment-based hypothesis that reveals the need for improved generalisability to tackle diversity present in multi-centre datasets.
FUSeg: The Foot Ulcer Segmentation Challenge
Jan 02, 2022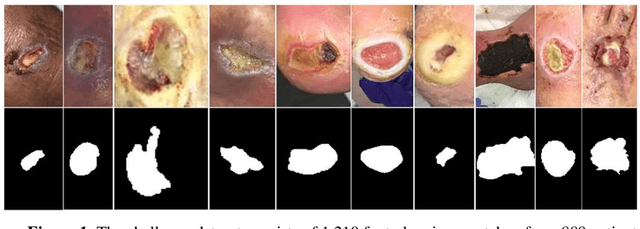
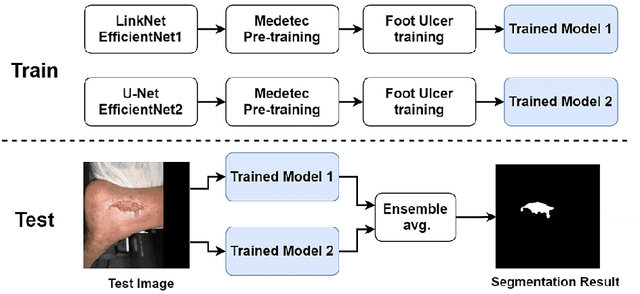
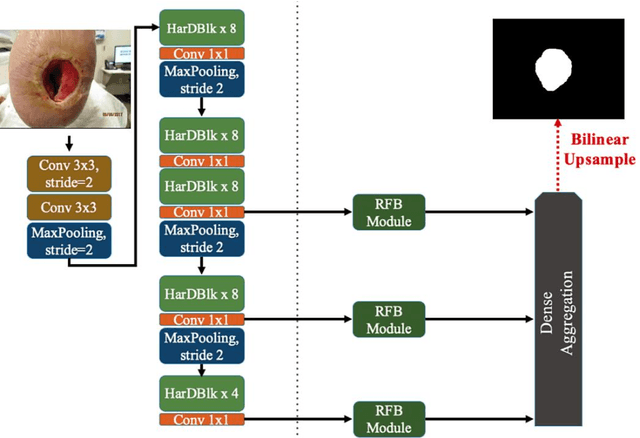
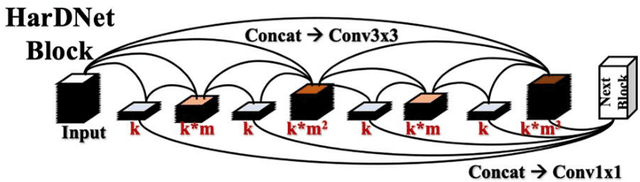
Abstract:Acute and chronic wounds with varying etiologies burden the healthcare systems economically. The advanced wound care market is estimated to reach $22 billion by 2024. Wound care professionals provide proper diagnosis and treatment with heavy reliance on images and image documentation. Segmentation of wound boundaries in images is a key component of the care and diagnosis protocol since it is important to estimate the area of the wound and provide quantitative measurement for the treatment. Unfortunately, this process is very time-consuming and requires a high level of expertise. Recently automatic wound segmentation methods based on deep learning have shown promising performance but require large datasets for training and it is unclear which methods perform better. To address these issues, we propose the Foot Ulcer Segmentation challenge (FUSeg) organized in conjunction with the 2021 International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI). We built a wound image dataset containing 1,210 foot ulcer images collected over 2 years from 889 patients. It is pixel-wise annotated by wound care experts and split into a training set with 1010 images and a testing set with 200 images for evaluation. Teams around the world developed automated methods to predict wound segmentations on the testing set of which annotations were kept private. The predictions were evaluated and ranked based on the average Dice coefficient. The FUSeg challenge remains an open challenge as a benchmark for wound segmentation after the conference.
The Devil is in the Margin: Margin-based Label Smoothing for Network Calibration
Nov 30, 2021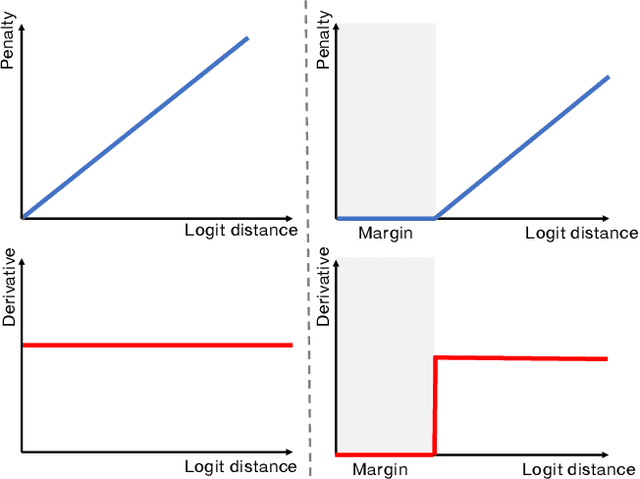
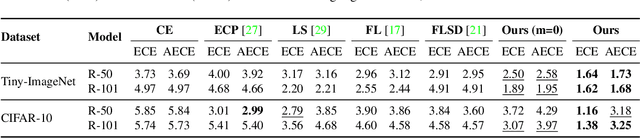
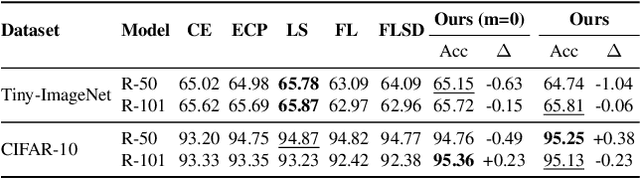
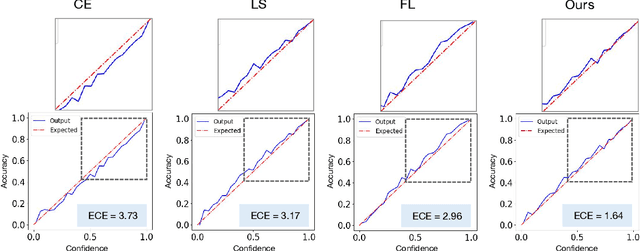
Abstract:In spite of the dominant performances of deep neural networks, recent works have shown that they are poorly calibrated, resulting in over-confident predictions. Miscalibration can be exacerbated by overfitting due to the minimization of the cross-entropy during training, as it promotes the predicted softmax probabilities to match the one-hot label assignments. This yields a pre-softmax activation of the correct class that is significantly larger than the remaining activations. Recent evidence from the literature suggests that loss functions that embed implicit or explicit maximization of the entropy of predictions yield state-of-the-art calibration performances. We provide a unifying constrained-optimization perspective of current state-of-the-art calibration losses. Specifically, these losses could be viewed as approximations of a linear penalty (or a Lagrangian) imposing equality constraints on logit distances. This points to an important limitation of such underlying equality constraints, whose ensuing gradients constantly push towards a non-informative solution, which might prevent from reaching the best compromise between the discriminative performance and calibration of the model during gradient-based optimization. Following our observations, we propose a simple and flexible generalization based on inequality constraints, which imposes a controllable margin on logit distances. Comprehensive experiments on a variety of image classification, semantic segmentation and NLP benchmarks demonstrate that our method sets novel state-of-the-art results on these tasks in terms of network calibration, without affecting the discriminative performance. The code is available at https://github.com/by-liu/MbLS .
A Hierarchical Multi-Task Approach to Gastrointestinal Image Analysis
Nov 16, 2021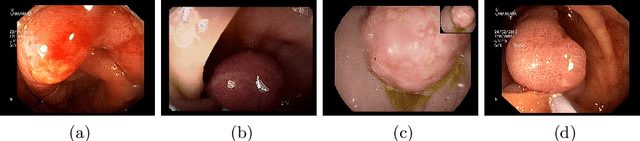
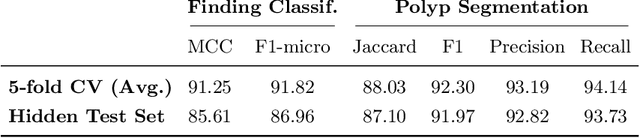
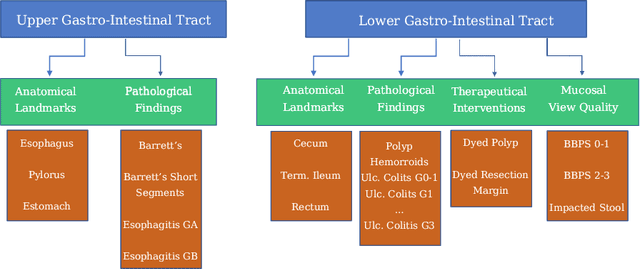
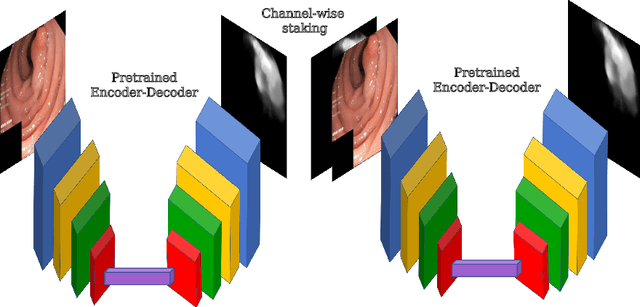
Abstract:A large number of different lesions and pathologies can affect the human digestive system, resulting in life-threatening situations. Early detection plays a relevant role in the successful treatment and the increase of current survival rates to, e.g., colorectal cancer. The standard procedure enabling detection, endoscopic video analysis, generates large quantities of visual data that need to be carefully analyzed by an specialist. Due to the wide range of color, shape, and general visual appearance of pathologies, as well as highly varying image quality, such process is greatly dependent on the human operator experience and skill. In this work, we detail our solution to the task of multi-category classification of images from the gastrointestinal (GI) human tract within the 2020 Endotect Challenge. Our approach is based on a Convolutional Neural Network minimizing a hierarchical error function that takes into account not only the finding category, but also its location within the GI tract (lower/upper tract), and the type of finding (pathological finding/therapeutic intervention/anatomical landmark/mucosal views' quality). We also describe in this paper our solution for the challenge task of polyp segmentation in colonoscopies, which was addressed with a pretrained double encoder-decoder network. Our internal cross-validation results show an average performance of 91.25 Mathews Correlation Coefficient (MCC) and 91.82 Micro-F1 score for the classification task, and a 92.30 F1 score for the polyp segmentation task. The organization provided feedback on the performance in a hidden test set for both tasks, which resulted in 85.61 MCC and 86.96 F1 score for classification, and 91.97 F1 score for polyp segmentation. At the time of writing no public ranking for this challenge had been released.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge