Yue Zeng
Zero-Shot Multi-modal Large Language Model v.s. Supervised Deep Learning: A Comparative Study on CT-Based Intracranial Hemorrhage Subtyping
May 14, 2025Abstract:Introduction: Timely identification of intracranial hemorrhage (ICH) subtypes on non-contrast computed tomography is critical for prognosis prediction and therapeutic decision-making, yet remains challenging due to low contrast and blurring boundaries. This study evaluates the performance of zero-shot multi-modal large language models (MLLMs) compared to traditional deep learning methods in ICH binary classification and subtyping. Methods: We utilized a dataset provided by RSNA, comprising 192 NCCT volumes. The study compares various MLLMs, including GPT-4o, Gemini 2.0 Flash, and Claude 3.5 Sonnet V2, with conventional deep learning models, including ResNet50 and Vision Transformer. Carefully crafted prompts were used to guide MLLMs in tasks such as ICH presence, subtype classification, localization, and volume estimation. Results: The results indicate that in the ICH binary classification task, traditional deep learning models outperform MLLMs comprehensively. For subtype classification, MLLMs also exhibit inferior performance compared to traditional deep learning models, with Gemini 2.0 Flash achieving an macro-averaged precision of 0.41 and a macro-averaged F1 score of 0.31. Conclusion: While MLLMs excel in interactive capabilities, their overall accuracy in ICH subtyping is inferior to deep networks. However, MLLMs enhance interpretability through language interactions, indicating potential in medical imaging analysis. Future efforts will focus on model refinement and developing more precise MLLMs to improve performance in three-dimensional medical image processing.
Graph Pseudotime Analysis and Neural Stochastic Differential Equations for Analyzing Retinal Degeneration Dynamics and Beyond
Feb 10, 2025Abstract:Understanding disease progression at the molecular pathway level usually requires capturing both structural dependencies between pathways and the temporal dynamics of disease evolution. In this work, we solve the former challenge by developing a biologically informed graph-forming method to efficiently construct pathway graphs for subjects from our newly curated JR5558 mouse transcriptomics dataset. We then develop Graph-level Pseudotime Analysis (GPA) to infer graph-level trajectories that reveal how disease progresses at the population level, rather than in individual subjects. Based on the trajectories estimated by GPA, we identify the most sensitive pathways that drive disease stage transitions. In addition, we measure changes in pathway features using neural stochastic differential equations (SDEs), which enables us to formally define and compute pathway stability and disease bifurcation points (points of no return), two fundamental problems in disease progression research. We further extend our theory to the case when pathways can interact with each other, enabling a more comprehensive and multi-faceted characterization of disease phenotypes. The comprehensive experimental results demonstrate the effectiveness of our framework in reconstructing the dynamics of the pathway, identifying critical transitions, and providing novel insights into the mechanistic understanding of disease evolution.
Machine Learning-Based Prediction of Key Genes Correlated to the Subretinal Lesion Severity in a Mouse Model of Age-Related Macular Degeneration
Sep 08, 2024



Abstract:Age-related macular degeneration (AMD) is a major cause of blindness in older adults, severely affecting vision and quality of life. Despite advances in understanding AMD, the molecular factors driving the severity of subretinal scarring (fibrosis) remain elusive, hampering the development of effective therapies. This study introduces a machine learning-based framework to predict key genes that are strongly correlated with lesion severity and to identify potential therapeutic targets to prevent subretinal fibrosis in AMD. Using an original RNA sequencing (RNA-seq) dataset from the diseased retinas of JR5558 mice, we developed a novel and specific feature engineering technique, including pathway-based dimensionality reduction and gene-based feature expansion, to enhance prediction accuracy. Two iterative experiments were conducted by leveraging Ridge and ElasticNet regression models to assess biological relevance and gene impact. The results highlight the biological significance of several key genes and demonstrate the framework's effectiveness in identifying novel therapeutic targets. The key findings provide valuable insights for advancing drug discovery efforts and improving treatment strategies for AMD, with the potential to enhance patient outcomes by targeting the underlying genetic mechanisms of subretinal lesion development.
Spatial--spectral FFPNet: Attention-Based Pyramid Network for Segmentation and Classification of Remote Sensing Images
Aug 20, 2020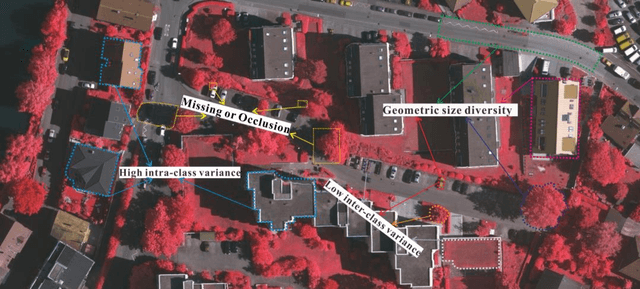
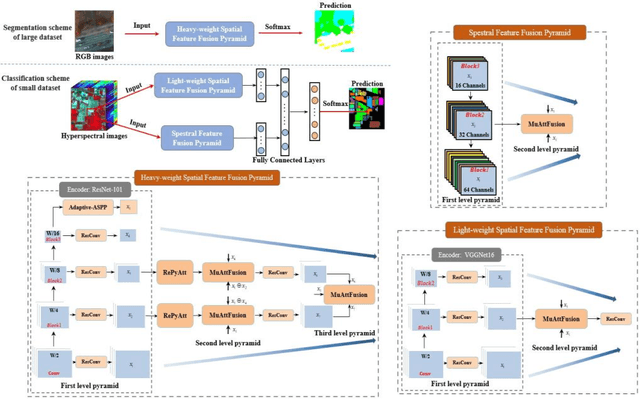
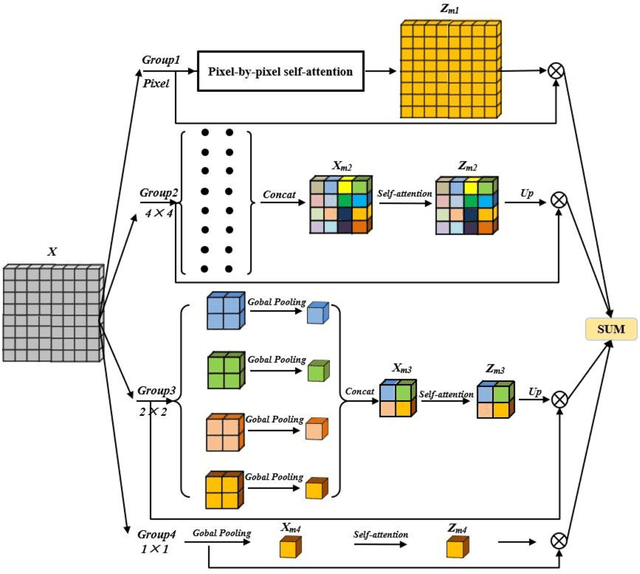
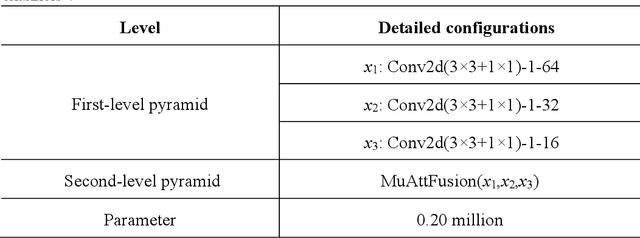
Abstract:We consider the problem of segmentation and classification of high-resolution and hyperspectral remote sensing images. Unlike conventional natural (RGB) images, the inherent large scale and complex structures of remote sensing images pose major challenges such as spatial object distribution diversity and spectral information extraction when existing models are directly applied for image classification. In this study, we develop an attention-based pyramid network for segmentation and classification of remote sensing datasets. Attention mechanisms are used to develop the following modules: i) a novel and robust attention-based multi-scale fusion method effectively fuses useful spatial or spectral information at different and same scales; ii) a region pyramid attention mechanism using region-based attention addresses the target geometric size diversity in large-scale remote sensing images; and iii cross-scale attention} in our adaptive atrous spatial pyramid pooling network adapts to varied contents in a feature-embedded space. Different forms of feature fusion pyramid frameworks are established by combining these attention-based modules. First, a novel segmentation framework, called the heavy-weight spatial feature fusion pyramid network (FFPNet), is proposed to address the spatial problem of high-resolution remote sensing images. Second, an end-to-end spatial--spectral FFPNet is presented for classifying hyperspectral images. Experiments conducted on ISPRS Vaihingen and ISPRS Potsdam high-resolution datasets demonstrate the competitive segmentation accuracy achieved by the proposed heavy-weight spatial FFPNet. Furthermore, experiments on the Indian Pines and the University of Pavia hyperspectral datasets indicate that the proposed spatial--spectral FFPNet outperforms the current state-of-the-art methods in hyperspectral image classification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge