Uffe Kock Wiil
Dual Model Deep Learning for Alzheimer Prognostication
Dec 22, 2025



Abstract:Disease modifying therapies for Alzheimer's disease demand precise timing decisions, yet current predictive models require longitudinal observations and provide no uncertainty quantification, rendering them impractical at the critical first visit when treatment decisions must be made. We developed PROGRESS (PRognostic Generalization from REsting Static Signatures), a dual-model deep learning framework that transforms a single baseline cerebrospinal fluid biomarker assessment into actionable prognostic estimates without requiring prior clinical history. The framework addresses two complementary clinical questions: a probabilistic trajectory network predicts individualized cognitive decline with calibrated uncertainty bounds achieving near-nominal coverage, enabling honest prognostic communication; and a deep survival model estimates time to conversion from mild cognitive impairment to dementia. Using data from over 3,000 participants across 43 Alzheimer's Disease Research Centers in the National Alzheimer's Coordinating Center database, PROGRESS substantially outperforms Cox proportional hazards, Random Survival Forests, and gradient boosting methods for survival prediction. Risk stratification identifies patient groups with seven-fold differences in conversion rates, enabling clinically meaningful treatment prioritization. Leave-one-center-out validation demonstrates robust generalizability, with survival discrimination remaining strong across held-out sites despite heterogeneous measurement conditions spanning four decades of assay technologies. By combining superior survival prediction with trustworthy trajectory uncertainty quantification, PROGRESS bridges the gap between biomarker measurement and personalized clinical decision-making.
MedVQA-TREE: A Multimodal Reasoning and Retrieval Framework for Sarcopenia Prediction
Aug 26, 2025Abstract:Accurate sarcopenia diagnosis via ultrasound remains challenging due to subtle imaging cues, limited labeled data, and the absence of clinical context in most models. We propose MedVQA-TREE, a multimodal framework that integrates a hierarchical image interpretation module, a gated feature-level fusion mechanism, and a novel multi-hop, multi-query retrieval strategy. The vision module includes anatomical classification, region segmentation, and graph-based spatial reasoning to capture coarse, mid-level, and fine-grained structures. A gated fusion mechanism selectively integrates visual features with textual queries, while clinical knowledge is retrieved through a UMLS-guided pipeline accessing PubMed and a sarcopenia-specific external knowledge base. MedVQA-TREE was trained and evaluated on two public MedVQA datasets (VQA-RAD and PathVQA) and a custom sarcopenia ultrasound dataset. The model achieved up to 99% diagnostic accuracy and outperformed previous state-of-the-art methods by over 10%. These results underscore the benefit of combining structured visual understanding with guided knowledge retrieval for effective AI-assisted diagnosis in sarcopenia.
Pulmonologists-Level lung cancer detection based on standard blood test results and smoking status using an explainable machine learning approach
Feb 14, 2024



Abstract:Lung cancer (LC) remains the primary cause of cancer-related mortality, largely due to late-stage diagnoses. Effective strategies for early detection are therefore of paramount importance. In recent years, machine learning (ML) has demonstrated considerable potential in healthcare by facilitating the detection of various diseases. In this retrospective development and validation study, we developed an ML model based on dynamic ensemble selection (DES) for LC detection. The model leverages standard blood sample analysis and smoking history data from a large population at risk in Denmark. The study includes all patients examined on suspicion of LC in the Region of Southern Denmark from 2009 to 2018. We validated and compared the predictions by the DES model with diagnoses provided by five pulmonologists. Among the 38,944 patients, 9,940 had complete data of which 2,505 (25\%) had LC. The DES model achieved an area under the roc curve of 0.77$\pm$0.01, sensitivity of 76.2\%$\pm$2.4\%, specificity of 63.8\%$\pm$2.3\%, positive predictive value of 41.6\%$\pm$1.2\%, and F\textsubscript{1}-score of 53.8\%$\pm$1.1\%. The DES model outperformed all five pulmonologists, achieving a sensitivity 9\% higher than their average. The model identified smoking status, age, total calcium levels, neutrophil count, and lactate dehydrogenase as the most important factors for the detection of LC. The results highlight the successful application of the ML approach in detecting LC, surpassing pulmonologists' performance. Incorporating clinical and laboratory data in future risk assessment models can improve decision-making and facilitate timely referrals.
Modeling Suspicious Email Detection using Enhanced Feature Selection
Dec 06, 2013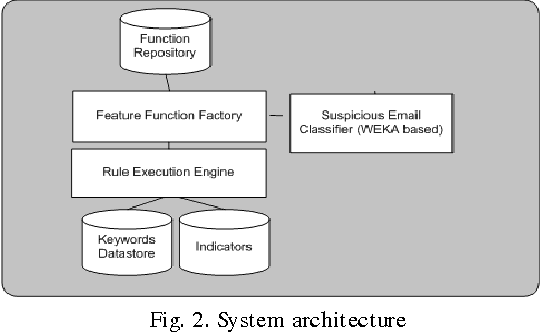
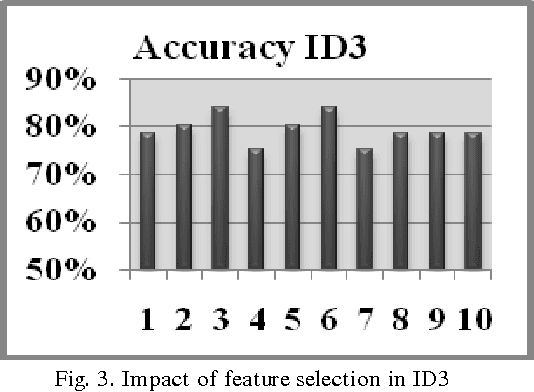
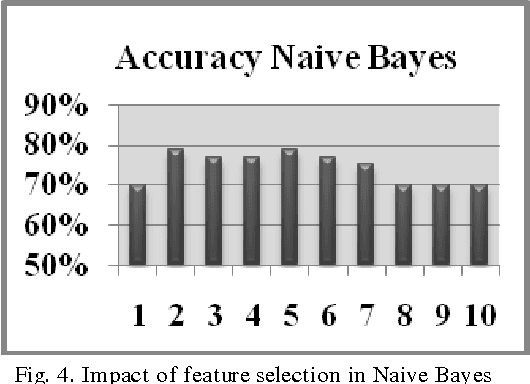
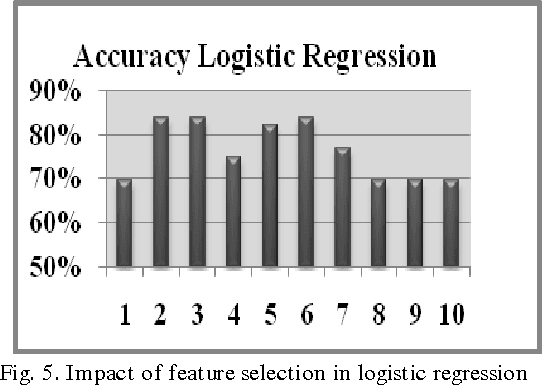
Abstract:The paper presents a suspicious email detection model which incorporates enhanced feature selection. In the paper we proposed the use of feature selection strategies along with classification technique for terrorists email detection. The presented model focuses on the evaluation of machine learning algorithms such as decision tree (ID3), logistic regression, Na\"ive Bayes (NB), and Support Vector Machine (SVM) for detecting emails containing suspicious content. In the literature, various algorithms achieved good accuracy for the desired task. However, the results achieved by those algorithms can be further improved by using appropriate feature selection mechanisms. We have identified the use of a specific feature selection scheme that improves the performance of the existing algorithms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge