Ruud B. van Heeswijk
Spatiotemporal graph neural process for reconstruction, extrapolation, and classification of cardiac trajectories
Sep 16, 2025



Abstract:We present a probabilistic framework for modeling structured spatiotemporal dynamics from sparse observations, focusing on cardiac motion. Our approach integrates neural ordinary differential equations (NODEs), graph neural networks (GNNs), and neural processes into a unified model that captures uncertainty, temporal continuity, and anatomical structure. We represent dynamic systems as spatiotemporal multiplex graphs and model their latent trajectories using a GNN-parameterized vector field. Given the sparse context observations at node and edge levels, the model infers a distribution over latent initial states and control variables, enabling both interpolation and extrapolation of trajectories. We validate the method on three synthetic dynamical systems (coupled pendulum, Lorenz attractor, and Kuramoto oscillators) and two real-world cardiac imaging datasets - ACDC (N=150) and UK Biobank (N=526) - demonstrating accurate reconstruction, extrapolation, and disease classification capabilities. The model accurately reconstructs trajectories and extrapolates future cardiac cycles from a single observed cycle. It achieves state-of-the-art results on the ACDC classification task (up to 99% accuracy), and detects atrial fibrillation in UK Biobank subjects with competitive performance (up to 67% accuracy). This work introduces a flexible approach for analyzing cardiac motion and offers a foundation for graph-based learning in structured biomedical spatiotemporal time-series data.
The R2D2 Deep Neural Network Series for Scalable Non-Cartesian Magnetic Resonance Imaging
Mar 13, 2025



Abstract:We introduce the R2D2 Deep Neural Network (DNN) series paradigm for fast and scalable image reconstruction from highly-accelerated non-Cartesian k-space acquisitions in Magnetic Resonance Imaging (MRI). While unrolled DNN architectures provide a robust image formation approach via data-consistency layers, embedding non-uniform fast Fourier transform operators in a DNN can become impractical to train at large scale, e.g in 2D MRI with a large number of coils, or for higher-dimensional imaging. Plug-and-play approaches that alternate a learned denoiser blind to the measurement setting with a data-consistency step are not affected by this limitation but their highly iterative nature implies slow reconstruction. To address this scalability challenge, we leverage the R2D2 paradigm that was recently introduced to enable ultra-fast reconstruction for large-scale Fourier imaging in radio astronomy. R2D2's reconstruction is formed as a series of residual images iteratively estimated as outputs of DNN modules taking the previous iteration's data residual as input. The method can be interpreted as a learned version of the Matching Pursuit algorithm. A series of R2D2 DNN modules were sequentially trained in a supervised manner on the fastMRI dataset and validated for 2D multi-coil MRI in simulation and on real data, targeting highly under-sampled radial k-space sampling. Results suggest that a series with only few DNNs achieves superior reconstruction quality over its unrolled incarnation R2D2-Net (whose training is also much less scalable), and over the state-of-the-art diffusion-based "Decomposed Diffusion Sampler" approach (also characterised by a slower reconstruction process).
Scalable Learning-Based Sampling Optimization for Compressive Dynamic MRI
Feb 20, 2019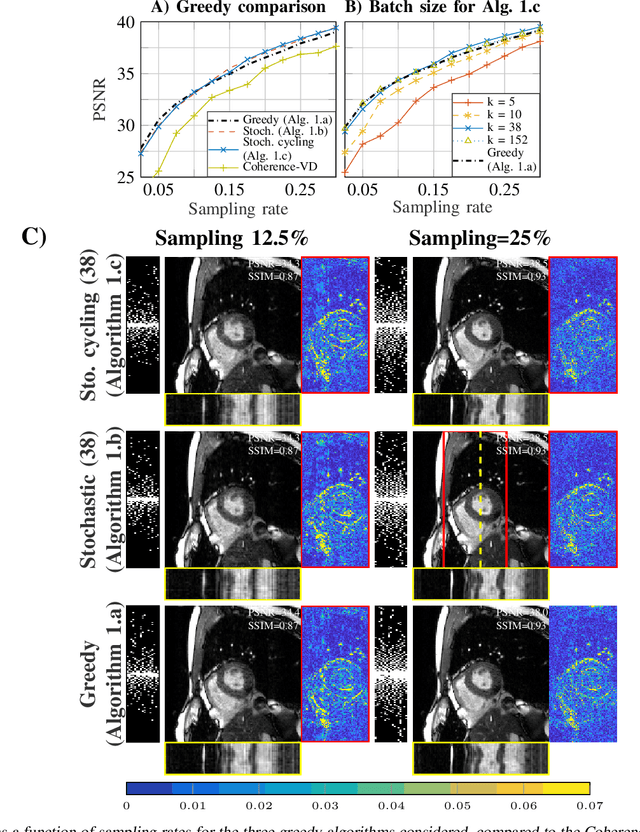
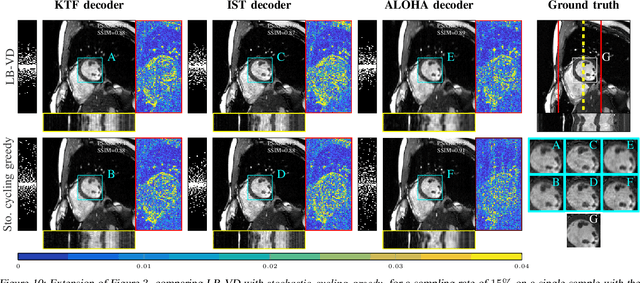
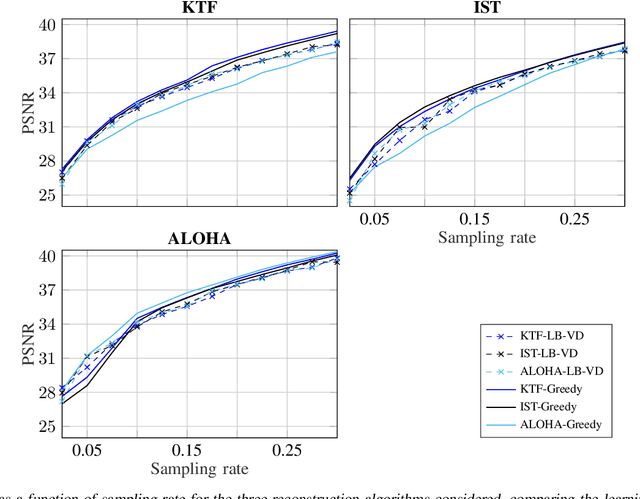
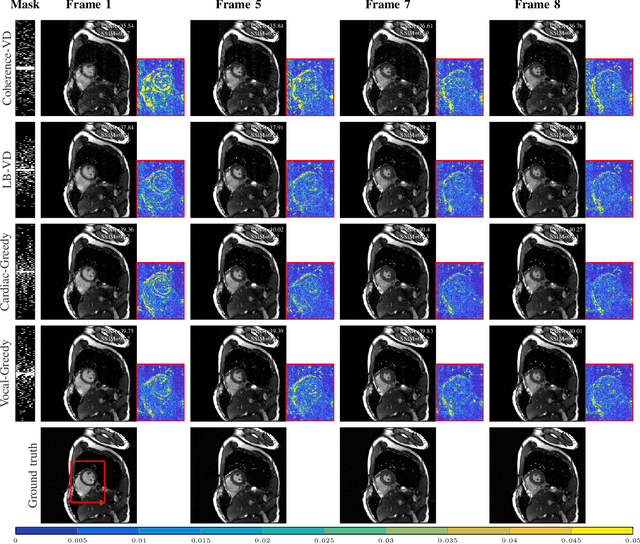
Abstract:Slow acquisition has been one of the historical problems in dynamic magnetic resonance imaging (dMRI), but the rise of compressed sensing (CS) has brought numerous algorithms that successfully achieve high acceleration rates. While CS proposes random sampling for data acquisition, practical CS applications to dMRI have typically relied on random variable-density (VD) sampling patterns, where masks are drawn from probabilistic models, which preferably sample from the center of the Fourier domain. In contrast to this model-driven approach, we propose the first data-driven, scalable framework for optimizing sampling patterns in dMRI. Through a greedy algorithm, this approach allows the data to directly govern the search for a mask that exhibits good empirical performance. Previous greedy approach, designed for static MRI, required very intensive computations, prohibiting their direct application to dMRI, and we address this issue by resorting to a stochastic greedy algorithm that exploits only a fraction of resources compared to the previous approach without sacrificing the reconstruction accuracy. A thorough comparison on in vivo datasets shows the inefficiency of model-based approaches in terms of sampling performance and suggests that our data-driven sampling approach could fully enable the potential of CS applied to dMRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge