Quande Liu
Dynamic Bank Learning for Semi-supervised Federated Image Diagnosis with Class Imbalance
Jun 27, 2022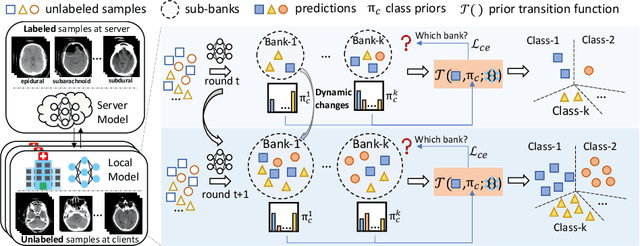
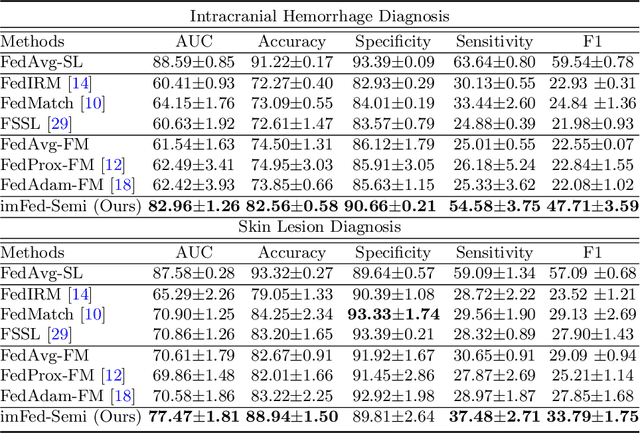
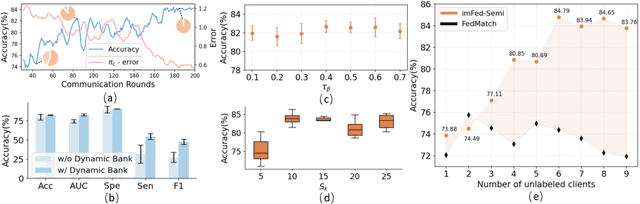
Abstract:Despite recent progress on semi-supervised federated learning (FL) for medical image diagnosis, the problem of imbalanced class distributions among unlabeled clients is still unsolved for real-world use. In this paper, we study a practical yet challenging problem of class imbalanced semi-supervised FL (imFed-Semi), which allows all clients to have only unlabeled data while the server just has a small amount of labeled data. This imFed-Semi problem is addressed by a novel dynamic bank learning scheme, which improves client training by exploiting class proportion information. This scheme consists of two parts, i.e., the dynamic bank construction to distill various class proportions for each local client, and the sub-bank classification to impose the local model to learn different class proportions. We evaluate our approach on two public real-world medical datasets, including the intracranial hemorrhage diagnosis with 25,000 CT slices and skin lesion diagnosis with 10,015 dermoscopy images. The effectiveness of our method has been validated with significant performance improvements (7.61% and 4.69%) compared with the second-best on the accuracy, as well as comprehensive analytical studies. Code is available at https://github.com/med-air/imFedSemi.
DLTTA: Dynamic Learning Rate for Test-time Adaptation on Cross-domain Medical Images
May 27, 2022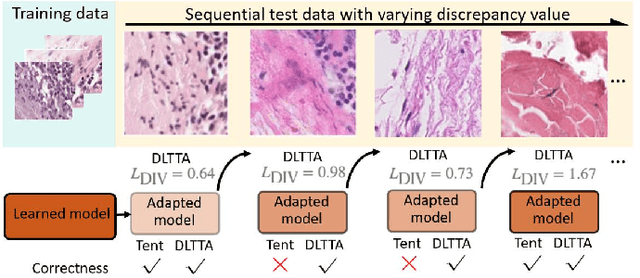
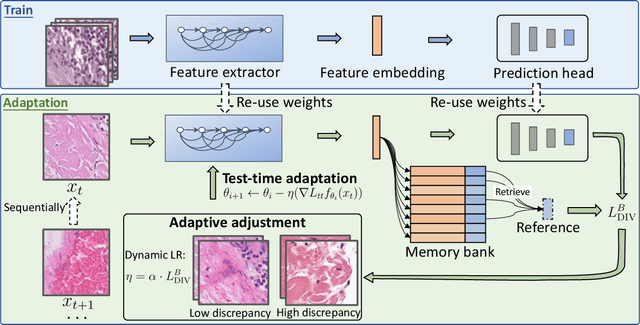
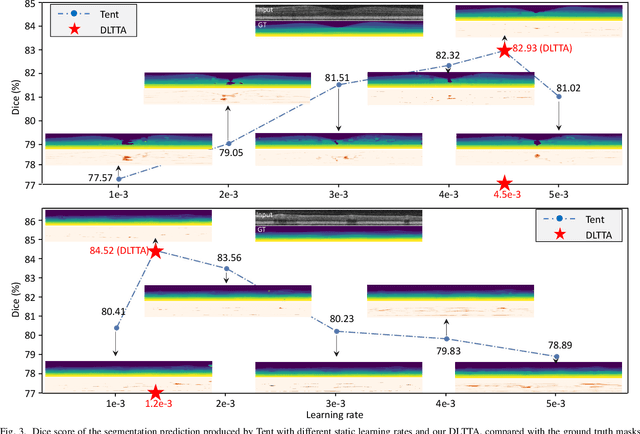
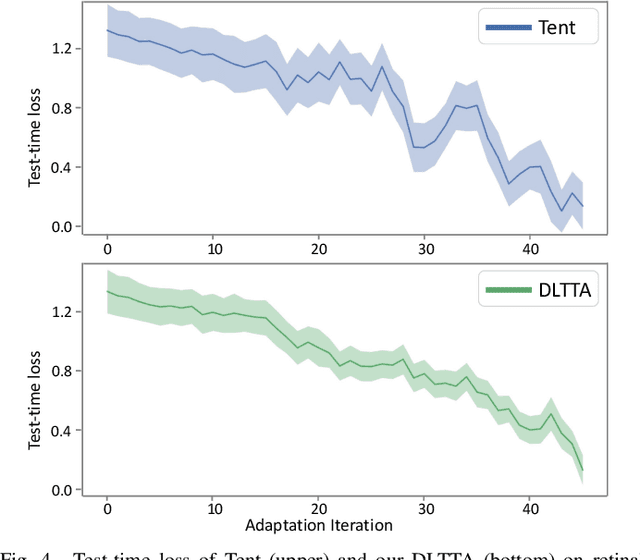
Abstract:Test-time adaptation (TTA) has increasingly been an important topic to efficiently tackle the cross-domain distribution shift at test time for medical images from different institutions. Previous TTA methods have a common limitation of using a fixed learning rate for all the test samples. Such a practice would be sub-optimal for TTA, because test data may arrive sequentially therefore the scale of distribution shift would change frequently. To address this problem, we propose a novel dynamic learning rate adjustment method for test-time adaptation, called DLTTA, which dynamically modulates the amount of weights update for each test image to account for the differences in their distribution shift. Specifically, our DLTTA is equipped with a memory bank based estimation scheme to effectively measure the discrepancy of a given test sample. Based on this estimated discrepancy, a dynamic learning rate adjustment strategy is then developed to achieve a suitable degree of adaptation for each test sample. The effectiveness and general applicability of our DLTTA is extensively demonstrated on three tasks including retinal optical coherence tomography (OCT) segmentation, histopathological image classification, and prostate 3D MRI segmentation. Our method achieves effective and fast test-time adaptation with consistent performance improvement over current state-of-the-art test-time adaptation methods. Code is available at: https://github.com/med-air/DLTTA.
Source-Free Domain Adaptive Fundus Image Segmentation with Denoised Pseudo-Labeling
Sep 19, 2021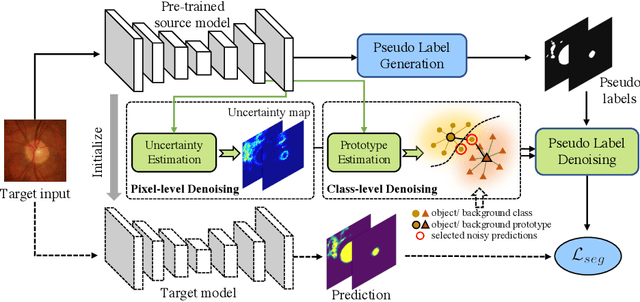
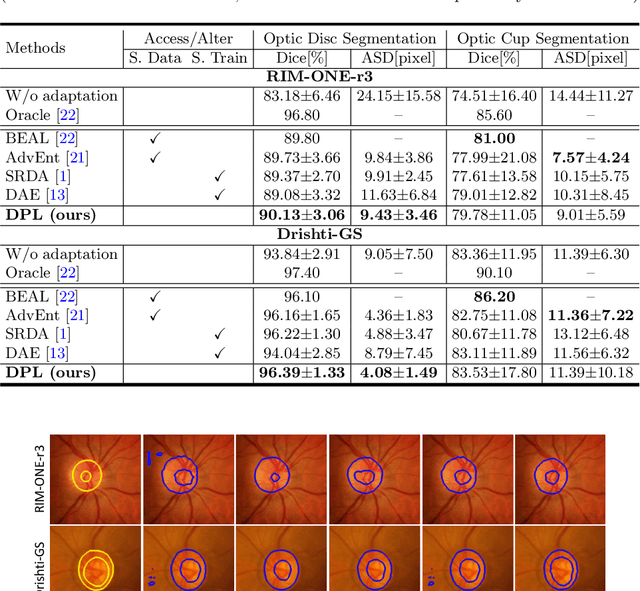
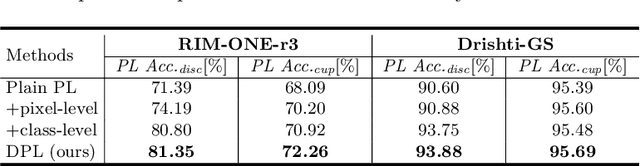
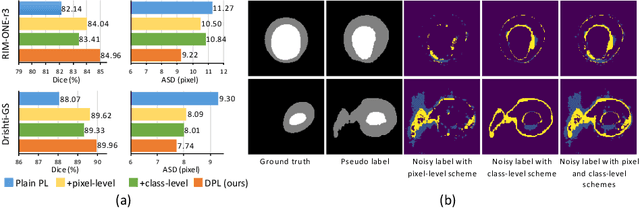
Abstract:Domain adaptation typically requires to access source domain data to utilize their distribution information for domain alignment with the target data. However, in many real-world scenarios, the source data may not be accessible during the model adaptation in the target domain due to privacy issue. This paper studies the practical yet challenging source-free unsupervised domain adaptation problem, in which only an existing source model and the unlabeled target data are available for model adaptation. We present a novel denoised pseudo-labeling method for this problem, which effectively makes use of the source model and unlabeled target data to promote model self-adaptation from pseudo labels. Importantly, considering that the pseudo labels generated from source model are inevitably noisy due to domain shift, we further introduce two complementary pixel-level and class-level denoising schemes with uncertainty estimation and prototype estimation to reduce noisy pseudo labels and select reliable ones to enhance the pseudo-labeling efficacy. Experimental results on cross-domain fundus image segmentation show that without using any source images or altering source training, our approach achieves comparable or even higher performance than state-of-the-art source-dependent unsupervised domain adaptation methods.
Federated Semi-supervised Medical Image Classification via Inter-client Relation Matching
Jun 16, 2021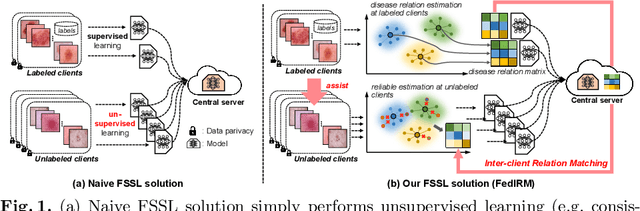
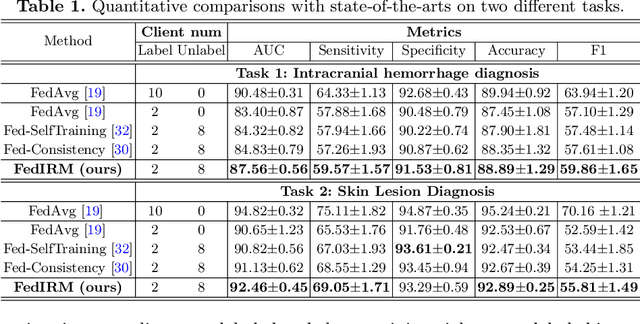
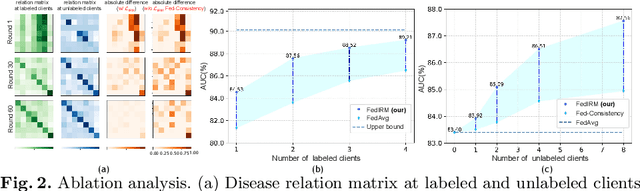
Abstract:Federated learning (FL) has emerged with increasing popularity to collaborate distributed medical institutions for training deep networks. However, despite existing FL algorithms only allow the supervised training setting, most hospitals in realistic usually cannot afford the intricate data labeling due to absence of budget or expertise. This paper studies a practical yet challenging FL problem, named \textit{Federated Semi-supervised Learning} (FSSL), which aims to learn a federated model by jointly utilizing the data from both labeled and unlabeled clients (i.e., hospitals). We present a novel approach for this problem, which improves over traditional consistency regularization mechanism with a new inter-client relation matching scheme. The proposed learning scheme explicitly connects the learning across labeled and unlabeled clients by aligning their extracted disease relationships, thereby mitigating the deficiency of task knowledge at unlabeled clients and promoting discriminative information from unlabeled samples. We validate our method on two large-scale medical image classification datasets. The effectiveness of our method has been demonstrated with the clear improvements over state-of-the-arts as well as the thorough ablation analysis on both tasks\footnote{Code will be made available at \url{https://github.com/liuquande/FedIRM}}.
FedDG: Federated Domain Generalization on Medical Image Segmentation via Episodic Learning in Continuous Frequency Space
Mar 10, 2021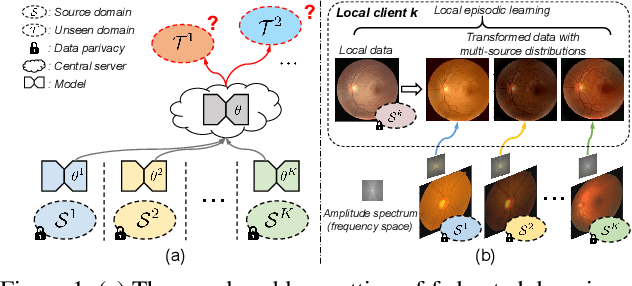
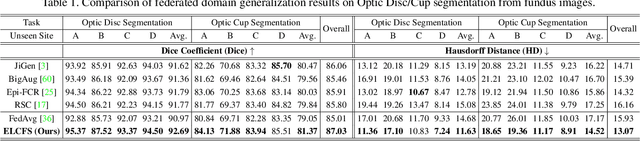
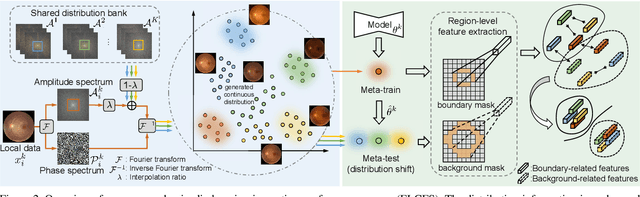
Abstract:Federated learning allows distributed medical institutions to collaboratively learn a shared prediction model with privacy protection. While at clinical deployment, the models trained in federated learning can still suffer from performance drop when applied to completely unseen hospitals outside the federation. In this paper, we point out and solve a novel problem setting of federated domain generalization (FedDG), which aims to learn a federated model from multiple distributed source domains such that it can directly generalize to unseen target domains. We present a novel approach, named as Episodic Learning in Continuous Frequency Space (ELCFS), for this problem by enabling each client to exploit multi-source data distributions under the challenging constraint of data decentralization. Our approach transmits the distribution information across clients in a privacy-protecting way through an effective continuous frequency space interpolation mechanism. With the transferred multi-source distributions, we further carefully design a boundary-oriented episodic learning paradigm to expose the local learning to domain distribution shifts and particularly meet the challenges of model generalization in medical image segmentation scenario. The effectiveness of our method is demonstrated with superior performance over state-of-the-arts and in-depth ablation experiments on two medical image segmentation tasks. The code is available at "https://github.com/liuquande/FedDG-ELCFS".
Contrastive Cross-site Learning with Redesigned Net for COVID-19 CT Classification
Sep 15, 2020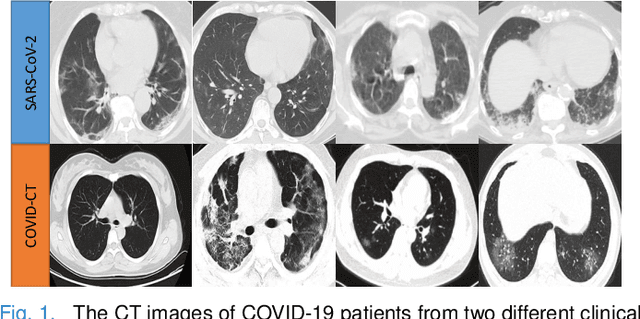
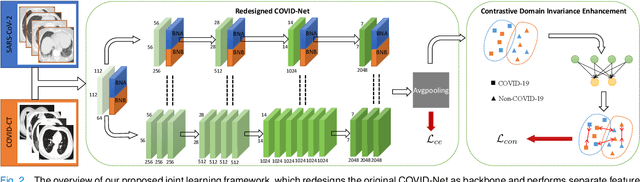
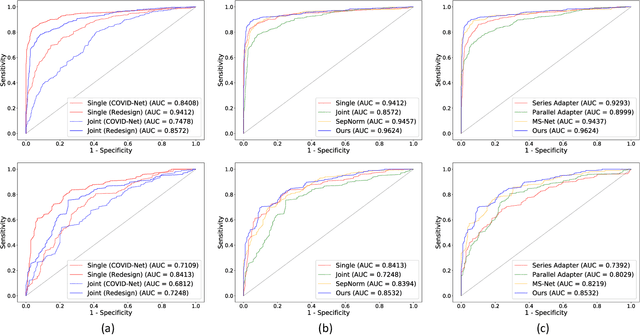
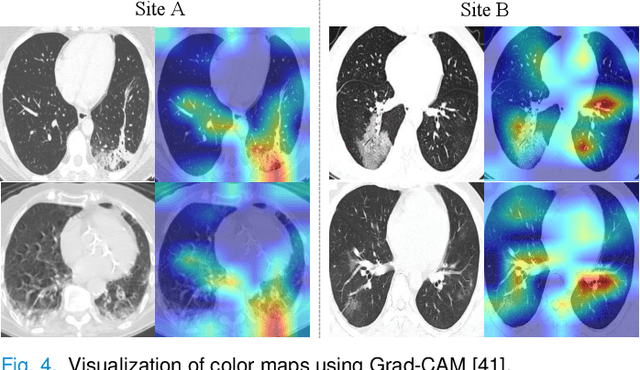
Abstract:The pandemic of coronavirus disease 2019 (COVID-19) has lead to a global public health crisis spreading hundreds of countries. With the continuous growth of new infections, developing automated tools for COVID-19 identification with CT image is highly desired to assist the clinical diagnosis and reduce the tedious workload of image interpretation. To enlarge the datasets for developing machine learning methods, it is essentially helpful to aggregate the cases from different medical systems for learning robust and generalizable models. This paper proposes a novel joint learning framework to perform accurate COVID-19 identification by effectively learning with heterogeneous datasets with distribution discrepancy. We build a powerful backbone by redesigning the recently proposed COVID-Net in aspects of network architecture and learning strategy to improve the prediction accuracy and learning efficiency. On top of our improved backbone, we further explicitly tackle the cross-site domain shift by conducting separate feature normalization in latent space. Moreover, we propose to use a contrastive training objective to enhance the domain invariance of semantic embeddings for boosting the classification performance on each dataset. We develop and evaluate our method with two public large-scale COVID-19 diagnosis datasets made up of CT images. Extensive experiments show that our approach consistently improves the performances on both datasets, outperforming the original COVID-Net trained on each dataset by 12.16% and 14.23% in AUC respectively, also exceeding existing state-of-the-art multi-site learning methods.
Shape-aware Meta-learning for Generalizing Prostate MRI Segmentation to Unseen Domains
Jul 04, 2020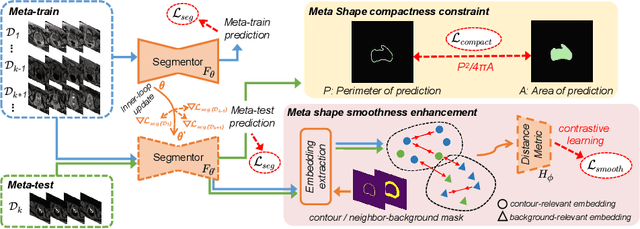
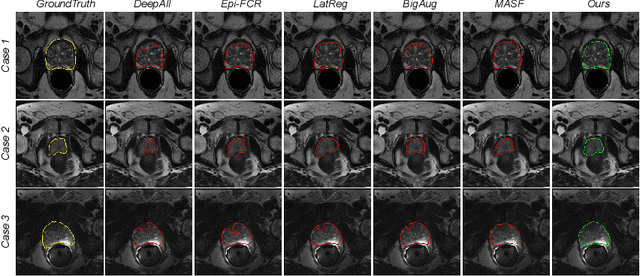
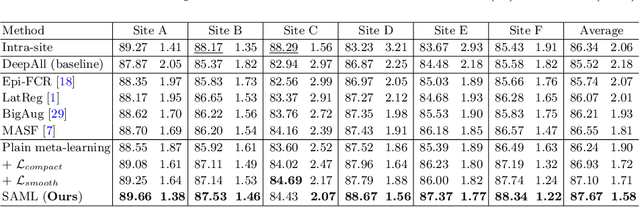
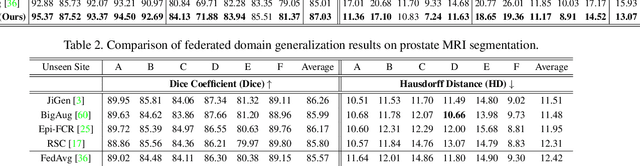
Abstract:Model generalization capacity at domain shift (e.g., various imaging protocols and scanners) is crucial for deep learning methods in real-world clinical deployment. This paper tackles the challenging problem of domain generalization, i.e., learning a model from multi-domain source data such that it can directly generalize to an unseen target domain. We present a novel shape-aware meta-learning scheme to improve the model generalization in prostate MRI segmentation. Our learning scheme roots in the gradient-based meta-learning, by explicitly simulating domain shift with virtual meta-train and meta-test during training. Importantly, considering the deficiencies encountered when applying a segmentation model to unseen domains (i.e., incomplete shape and ambiguous boundary of the prediction masks), we further introduce two complementary loss objectives to enhance the meta-optimization, by particularly encouraging the shape compactness and shape smoothness of the segmentations under simulated domain shift. We evaluate our method on prostate MRI data from six different institutions with distribution shifts acquired from public datasets. Experimental results show that our approach outperforms many state-of-the-art generalization methods consistently across all six settings of unseen domains.
Deep Mining External Imperfect Data for Chest X-ray Disease Screening
Jun 06, 2020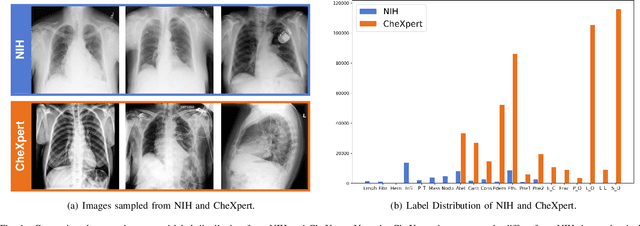
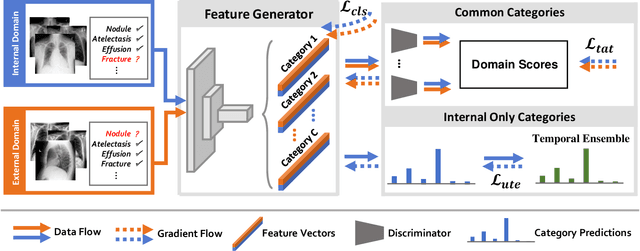
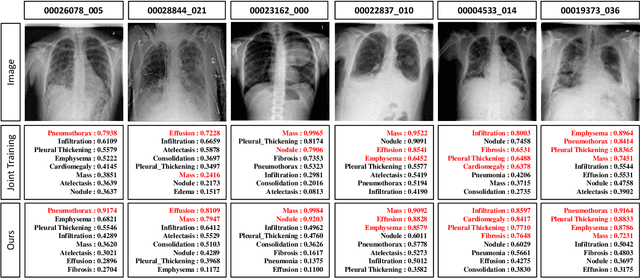
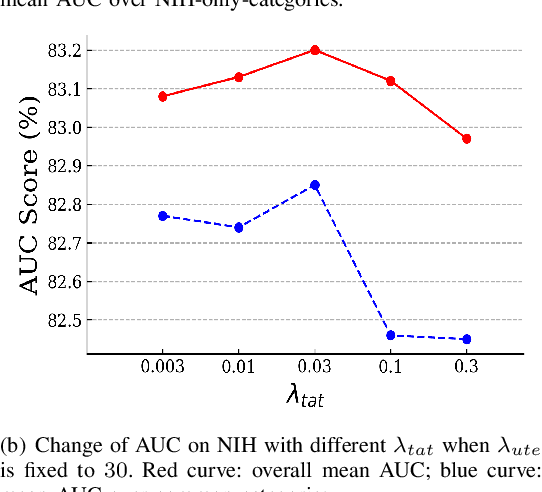
Abstract:Deep learning approaches have demonstrated remarkable progress in automatic Chest X-ray analysis. The data-driven feature of deep models requires training data to cover a large distribution. Therefore, it is substantial to integrate knowledge from multiple datasets, especially for medical images. However, learning a disease classification model with extra Chest X-ray (CXR) data is yet challenging. Recent researches have demonstrated that performance bottleneck exists in joint training on different CXR datasets, and few made efforts to address the obstacle. In this paper, we argue that incorporating an external CXR dataset leads to imperfect training data, which raises the challenges. Specifically, the imperfect data is in two folds: domain discrepancy, as the image appearances vary across datasets; and label discrepancy, as different datasets are partially labeled. To this end, we formulate the multi-label thoracic disease classification problem as weighted independent binary tasks according to the categories. For common categories shared across domains, we adopt task-specific adversarial training to alleviate the feature differences. For categories existing in a single dataset, we present uncertainty-aware temporal ensembling of model predictions to mine the information from the missing labels further. In this way, our framework simultaneously models and tackles the domain and label discrepancies, enabling superior knowledge mining ability. We conduct extensive experiments on three datasets with more than 360,000 Chest X-ray images. Our method outperforms other competing models and sets state-of-the-art performance on the official NIH test set with 0.8349 AUC, demonstrating its effectiveness of utilizing the external dataset to improve the internal classification.
Semi-supervised Medical Image Classification with Relation-driven Self-ensembling Model
May 15, 2020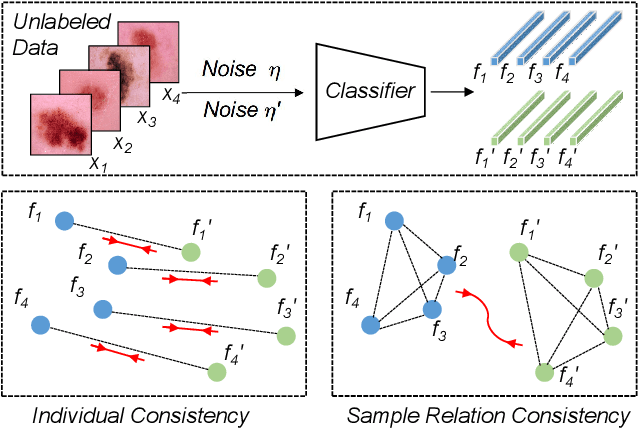
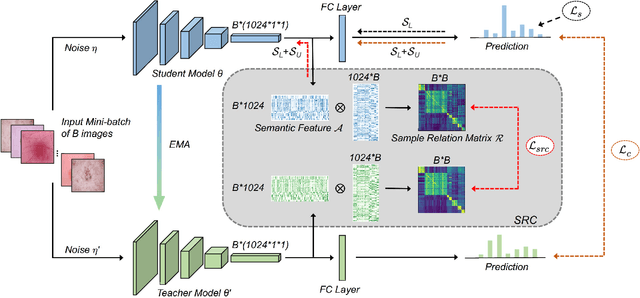
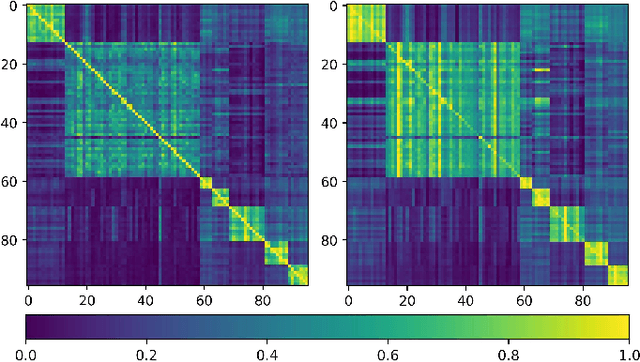
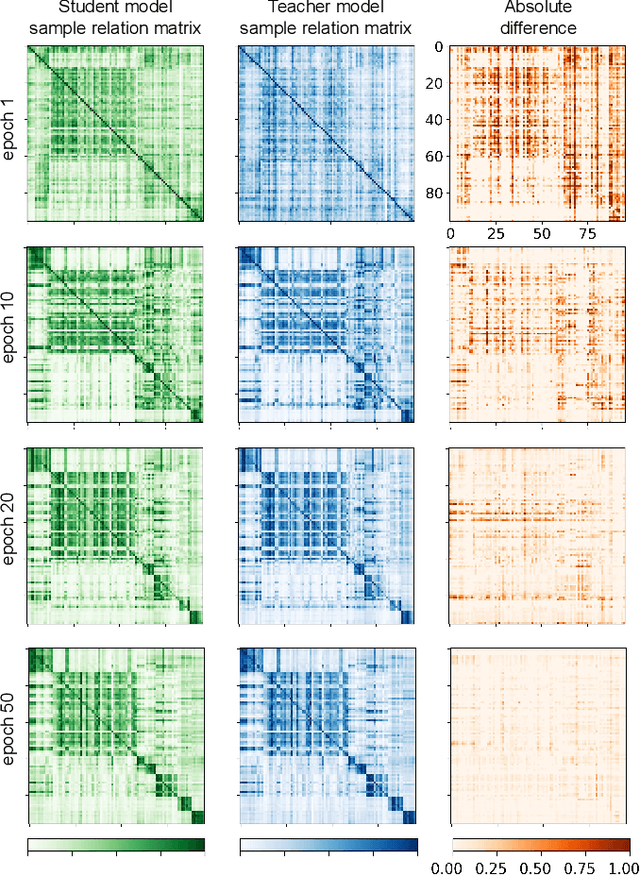
Abstract:Training deep neural networks usually requires a large amount of labeled data to obtain good performance. However, in medical image analysis, obtaining high-quality labels for the data is laborious and expensive, as accurately annotating medical images demands expertise knowledge of the clinicians. In this paper, we present a novel relation-driven semi-supervised framework for medical image classification. It is a consistency-based method which exploits the unlabeled data by encouraging the prediction consistency of given input under perturbations, and leverages a self-ensembling model to produce high-quality consistency targets for the unlabeled data. Considering that human diagnosis often refers to previous analogous cases to make reliable decisions, we introduce a novel sample relation consistency (SRC) paradigm to effectively exploit unlabeled data by modeling the relationship information among different samples. Superior to existing consistency-based methods which simply enforce consistency of individual predictions, our framework explicitly enforces the consistency of semantic relation among different samples under perturbations, encouraging the model to explore extra semantic information from unlabeled data. We have conducted extensive experiments to evaluate our method on two public benchmark medical image classification datasets, i.e.,skin lesion diagnosis with ISIC 2018 challenge and thorax disease classification with ChestX-ray14. Our method outperforms many state-of-the-art semi-supervised learning methods on both single-label and multi-label image classification scenarios.
MS-Net: Multi-Site Network for Improving Prostate Segmentation with Heterogeneous MRI Data
Feb 19, 2020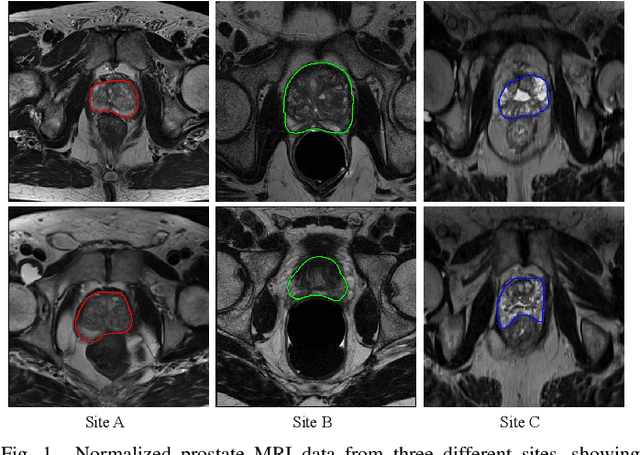
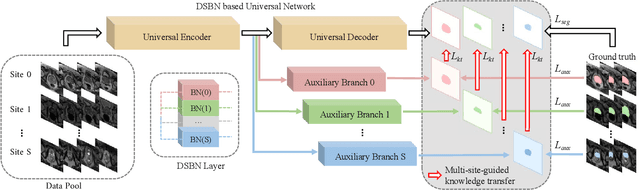
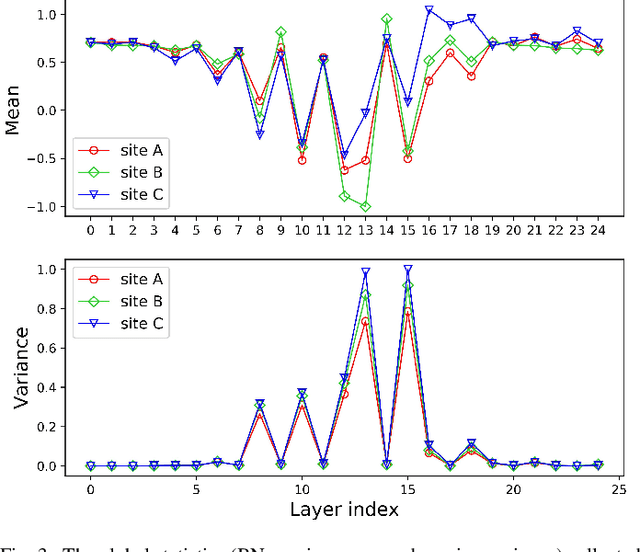
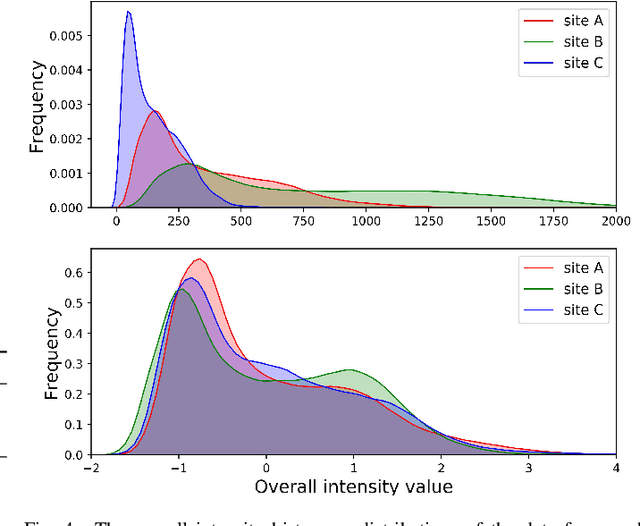
Abstract:Automated prostate segmentation in MRI is highly demanded for computer-assisted diagnosis. Recently, a variety of deep learning methods have achieved remarkable progress in this task, usually relying on large amounts of training data. Due to the nature of scarcity for medical images, it is important to effectively aggregate data from multiple sites for robust model training, to alleviate the insufficiency of single-site samples. However, the prostate MRIs from different sites present heterogeneity due to the differences in scanners and imaging protocols, raising challenges for effective ways of aggregating multi-site data for network training. In this paper, we propose a novel multi-site network (MS-Net) for improving prostate segmentation by learning robust representations, leveraging multiple sources of data. To compensate for the inter-site heterogeneity of different MRI datasets, we develop Domain-Specific Batch Normalization layers in the network backbone, enabling the network to estimate statistics and perform feature normalization for each site separately. Considering the difficulty of capturing the shared knowledge from multiple datasets, a novel learning paradigm, i.e., Multi-site-guided Knowledge Transfer, is proposed to enhance the kernels to extract more generic representations from multi-site data. Extensive experiments on three heterogeneous prostate MRI datasets demonstrate that our MS-Net improves the performance across all datasets consistently, and outperforms state-of-the-art methods for multi-site learning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge