Jiecong Lin
Ctrl-DNA: Controllable Cell-Type-Specific Regulatory DNA Design via Constrained RL
May 26, 2025Abstract:Designing regulatory DNA sequences that achieve precise cell-type-specific gene expression is crucial for advancements in synthetic biology, gene therapy and precision medicine. Although transformer-based language models (LMs) can effectively capture patterns in regulatory DNA, their generative approaches often struggle to produce novel sequences with reliable cell-specific activity. Here, we introduce Ctrl-DNA, a novel constrained reinforcement learning (RL) framework tailored for designing regulatory DNA sequences with controllable cell-type specificity. By formulating regulatory sequence design as a biologically informed constrained optimization problem, we apply RL to autoregressive genomic LMs, enabling the models to iteratively refine sequences that maximize regulatory activity in targeted cell types while constraining off-target effects. Our evaluation on human promoters and enhancers demonstrates that Ctrl-DNA consistently outperforms existing generative and RL-based approaches, generating high-fitness regulatory sequences and achieving state-of-the-art cell-type specificity. Moreover, Ctrl-DNA-generated sequences capture key cell-type-specific transcription factor binding sites (TFBS), short DNA motifs recognized by regulatory proteins that control gene expression, demonstrating the biological plausibility of the generated sequences.
EGFI: Drug-Drug Interaction Extraction and Generation with Fusion of Enriched Entity and Sentence Information
Jan 25, 2021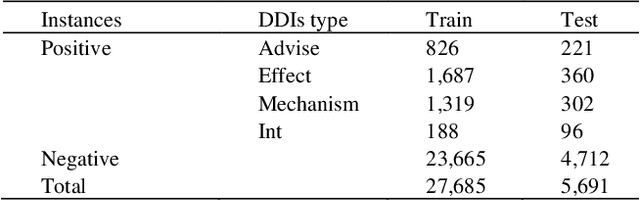
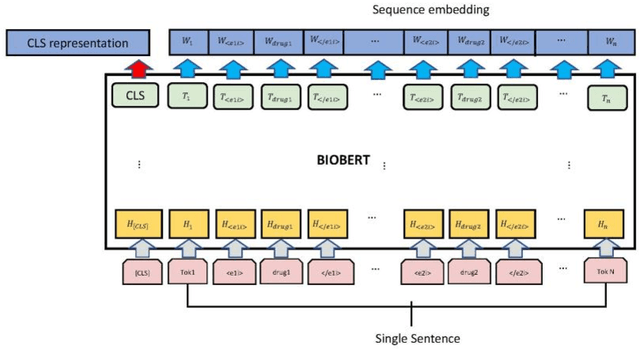
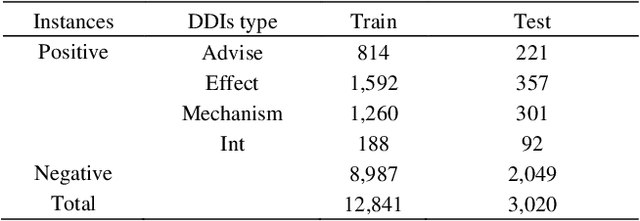
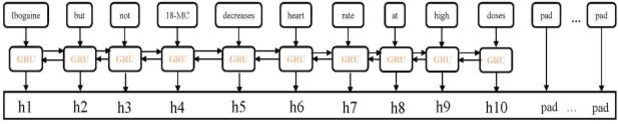
Abstract:The rapid growth in literature accumulates diverse and yet comprehensive biomedical knowledge hidden to be mined such as drug interactions. However, it is difficult to extract the heterogeneous knowledge to retrieve or even discover the latest and novel knowledge in an efficient manner. To address such a problem, we propose EGFI for extracting and consolidating drug interactions from large-scale medical literature text data. Specifically, EGFI consists of two parts: classification and generation. In the classification part, EGFI encompasses the language model BioBERT which has been comprehensively pre-trained on biomedical corpus. In particular, we propose the multi-head attention mechanism and pack BiGRU to fuse multiple semantic information for rigorous context modeling. In the generation part, EGFI utilizes another pre-trained language model BioGPT-2 where the generation sentences are selected based on filtering rules. We evaluated the classification part on "DDIs 2013" dataset and "DTIs" dataset, achieving the FI score of 0.842 and 0.720 respectively. Moreover, we applied the classification part to distinguish high-quality generated sentences and verified with the exiting growth truth to confirm the filtered sentences. The generated sentences that are not recorded in DrugBank and DDIs 2013 dataset also demonstrate the potential of EGFI to identify novel drug relationships.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge