Jan Ernsting
Towards Population Scale Testis Volume Segmentation in DIXON MRI
Oct 30, 2024Abstract:Testis size is known to be one of the main predictors of male fertility, usually assessed in clinical workup via palpation or imaging. Despite its potential, population-level evaluation of testicular volume using imaging remains underexplored. Previous studies, limited by small and biased datasets, have demonstrated the feasibility of machine learning for testis volume segmentation. This paper presents an evaluation of segmentation methods for testicular volume using Magnet Resonance Imaging data from the UKBiobank. The best model achieves a median dice score of $0.87$, compared to median dice score of $0.83$ for human interrater reliability on the same dataset, enabling large-scale annotation on a population scale for the first time. Our overall aim is to provide a trained model, comparative baseline methods, and annotated training data to enhance accessibility and reproducibility in testis MRI segmentation research.
deepmriprep: Voxel-based Morphometry (VBM) Preprocessing via Deep Neural Networks
Aug 20, 2024



Abstract:Voxel-based Morphometry (VBM) has emerged as a powerful approach in neuroimaging research, utilized in over 7,000 studies since the year 2000. Using Magnetic Resonance Imaging (MRI) data, VBM assesses variations in the local density of brain tissue and examines its associations with biological and psychometric variables. Here, we present deepmriprep, a neural network-based pipeline that performs all necessary preprocessing steps for VBM analysis of T1-weighted MR images using deep neural networks. Utilizing the Graphics Processing Unit (GPU), deepmriprep is 37 times faster than CAT12, the leading VBM preprocessing toolbox. The proposed method matches CAT12 in accuracy for tissue segmentation and image registration across more than 100 datasets and shows strong correlations in VBM results. Tissue segmentation maps from deepmriprep have over 95% agreement with ground truth maps, and its non-linear registration, using supervised SYMNet, predicts smooth deformation fields comparable to CAT12. The high processing speed of deepmriprep enables rapid preprocessing of extensive datasets and thereby fosters the application of VBM analysis to large-scale neuroimaging studies and opens the door to real-time applications. Finally, deepmripreps straightforward, modular design enables researchers to easily understand, reuse, and advance the underlying methods, fostering further advancements in neuroimaging research. deepmriprep can be conveniently installed as a Python package and is publicly accessible at https://github.com/wwu-mmll/deepmriprep.
pyAKI -- An Open Source Solution to Automated KDIGO classification
Jan 23, 2024Abstract:Acute Kidney Injury (AKI) is a frequent complication in critically ill patients, affecting up to 50% of patients in the intensive care units. The lack of standardized and open-source tools for applying the Kidney Disease Improving Global Outcomes (KDIGO) criteria to time series data has a negative impact on workload and study quality. This project introduces pyAKI, an open-source pipeline addressing this gap by providing a comprehensive solution for consistent KDIGO criteria implementation. The pyAKI pipeline was developed and validated using a subset of the Medical Information Mart for Intensive Care (MIMIC)-IV database, a commonly used database in critical care research. We defined a standardized data model in order to ensure reproducibility. Validation against expert annotations demonstrated pyAKI's robust performance in implementing KDIGO criteria. Comparative analysis revealed its ability to surpass the quality of human labels. This work introduces pyAKI as an open-source solution for implementing the KDIGO criteria for AKI diagnosis using time series data with high accuracy and performance.
GateNet: A novel Neural Network Architecture for Automated Flow Cytometry Gating
Dec 12, 2023



Abstract:Flow cytometry is widely used to identify cell populations in patient-derived fluids such as peripheral blood (PB) or cerebrospinal fluid (CSF). While ubiquitous in research and clinical practice, flow cytometry requires gating, i.e. cell type identification which requires labor-intensive and error-prone manual adjustments. To facilitate this process, we designed GateNet, the first neural network architecture enabling full end-to-end automated gating without the need to correct for batch effects. We train GateNet with over 8,000,000 events based on N=127 PB and CSF samples which were manually labeled independently by four experts. We show that for novel, unseen samples, GateNet achieves human-level performance (F1 score ranging from 0.910 to 0.997). In addition we apply GateNet to a publicly available dataset confirming generalization with an F1 score of 0.936. As our implementation utilizes graphics processing units (GPU), gating only needs 15 microseconds per event. Importantly, we also show that GateNet only requires ~10 samples to reach human-level performance, rendering it widely applicable in all domains of flow cytometry.
DenseNet and Support Vector Machine classifications of major depressive disorder using vertex-wise cortical features
Nov 18, 2023



Abstract:Major depressive disorder (MDD) is a complex psychiatric disorder that affects the lives of hundreds of millions of individuals around the globe. Even today, researchers debate if morphological alterations in the brain are linked to MDD, likely due to the heterogeneity of this disorder. The application of deep learning tools to neuroimaging data, capable of capturing complex non-linear patterns, has the potential to provide diagnostic and predictive biomarkers for MDD. However, previous attempts to demarcate MDD patients and healthy controls (HC) based on segmented cortical features via linear machine learning approaches have reported low accuracies. In this study, we used globally representative data from the ENIGMA-MDD working group containing an extensive sample of people with MDD (N=2,772) and HC (N=4,240), which allows a comprehensive analysis with generalizable results. Based on the hypothesis that integration of vertex-wise cortical features can improve classification performance, we evaluated the classification of a DenseNet and a Support Vector Machine (SVM), with the expectation that the former would outperform the latter. As we analyzed a multi-site sample, we additionally applied the ComBat harmonization tool to remove potential nuisance effects of site. We found that both classifiers exhibited close to chance performance (balanced accuracy DenseNet: 51%; SVM: 53%), when estimated on unseen sites. Slightly higher classification performance (balanced accuracy DenseNet: 58%; SVM: 55%) was found when the cross-validation folds contained subjects from all sites, indicating site effect. In conclusion, the integration of vertex-wise morphometric features and the use of the non-linear classifier did not lead to the differentiability between MDD and HC. Our results support the notion that MDD classification on this combination of features and classifiers is unfeasible.
Deepbet: Fast brain extraction of T1-weighted MRI using Convolutional Neural Networks
Aug 14, 2023



Abstract:Brain extraction in magnetic resonance imaging (MRI) data is an important segmentation step in many neuroimaging preprocessing pipelines. Image segmentation is one of the research fields in which deep learning had the biggest impact in recent years enabling high precision segmentation with minimal compute. Consequently, traditional brain extraction methods are now being replaced by deep learning-based methods. Here, we used a unique dataset comprising 568 T1-weighted (T1w) MR images from 191 different studies in combination with cutting edge deep learning methods to build a fast, high-precision brain extraction tool called deepbet. deepbet uses LinkNet, a modern UNet architecture, in a two stage prediction process. This increases its segmentation performance, setting a novel state-of-the-art performance during cross-validation with a median Dice score (DSC) of 99.0% on unseen datasets, outperforming current state of the art models (DSC = 97.8% and DSC = 97.9%). While current methods are more sensitive to outliers, resulting in Dice scores as low as 76.5%, deepbet manages to achieve a Dice score of > 96.9% for all samples. Finally, our model accelerates brain extraction by a factor of ~10 compared to current methods, enabling the processing of one image in ~2 seconds on low level hardware.
From Group-Differences to Single-Subject Probability: Conformal Prediction-based Uncertainty Estimation for Brain-Age Modeling
Feb 10, 2023
Abstract:The brain-age gap is one of the most investigated risk markers for brain changes across disorders. While the field is progressing towards large-scale models, recently incorporating uncertainty estimates, no model to date provides the single-subject risk assessment capability essential for clinical application. In order to enable the clinical use of brain-age as a biomarker, we here combine uncertainty-aware deep Neural Networks with conformal prediction theory. This approach provides statistical guarantees with respect to single-subject uncertainty estimates and allows for the calculation of an individual's probability for accelerated brain-aging. Building on this, we show empirically in a sample of N=16,794 participants that 1. a lower or comparable error as state-of-the-art, large-scale brain-age models, 2. the statistical guarantees regarding single-subject uncertainty estimation indeed hold for every participant, and 3. that the higher individual probabilities of accelerated brain-aging derived from our model are associated with Alzheimer's Disease, Bipolar Disorder and Major Depressive Disorder.
An Uncertainty-Aware, Shareable and Transparent Neural Network Architecture for Brain-Age Modeling
Jul 16, 2021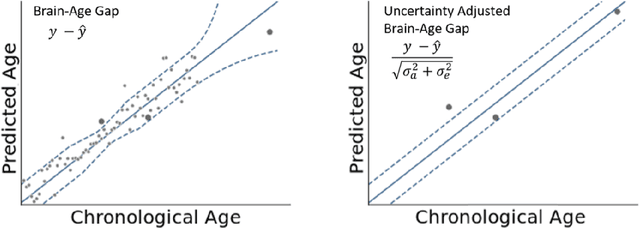
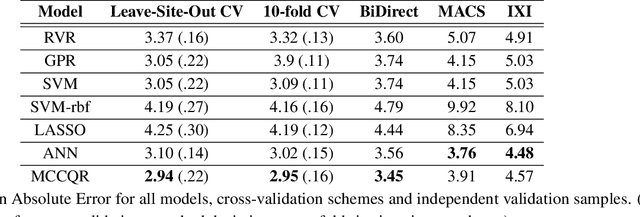
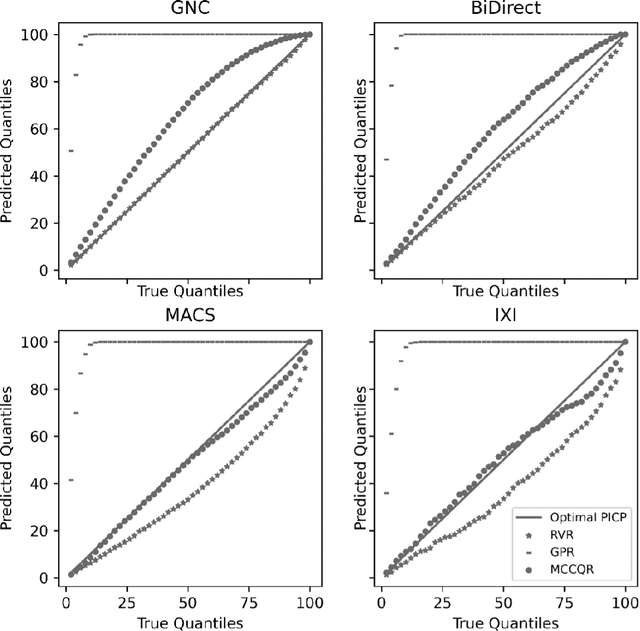
Abstract:The deviation between chronological age and age predicted from neuroimaging data has been identified as a sensitive risk-marker of cross-disorder brain changes, growing into a cornerstone of biological age-research. However, Machine Learning models underlying the field do not consider uncertainty, thereby confounding results with training data density and variability. Also, existing models are commonly based on homogeneous training sets, often not independently validated, and cannot be shared due to data protection issues. Here, we introduce an uncertainty-aware, shareable, and transparent Monte-Carlo Dropout Composite-Quantile-Regression (MCCQR) Neural Network trained on N=10,691 datasets from the German National Cohort. The MCCQR model provides robust, distribution-free uncertainty quantification in high-dimensional neuroimaging data, achieving lower error rates compared to existing models across ten recruitment centers and in three independent validation samples (N=4,004). In two examples, we demonstrate that it prevents spurious associations and increases power to detect accelerated brain-aging. We make the pre-trained model publicly available.
Predicting brain-age from raw T 1 -weighted Magnetic Resonance Imaging data using 3D Convolutional Neural Networks
Mar 22, 2021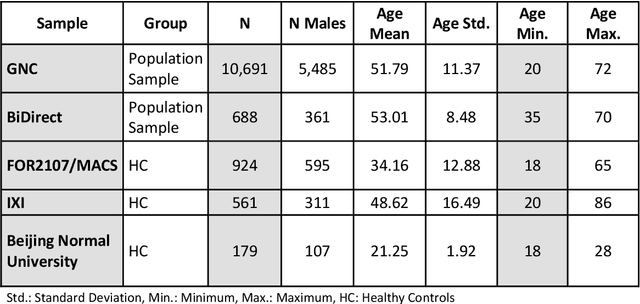
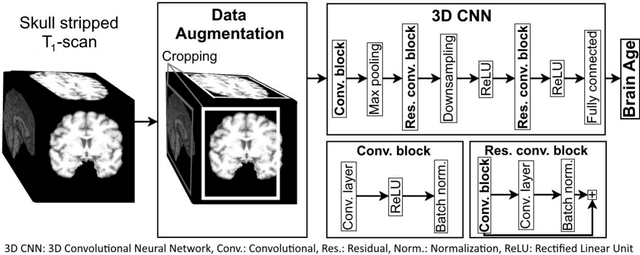
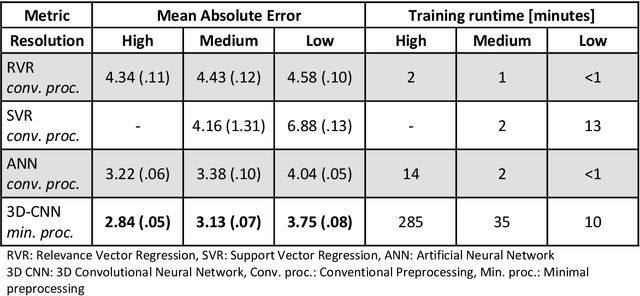
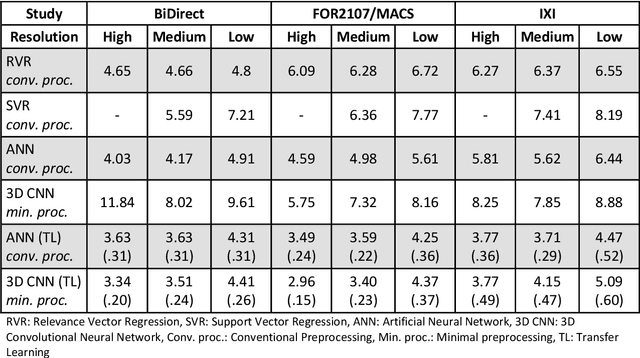
Abstract:Age prediction based on Magnetic Resonance Imaging (MRI) data of the brain is a biomarker to quantify the progress of brain diseases and aging. Current approaches rely on preparing the data with multiple preprocessing steps, such as registering voxels to a standardized brain atlas, which yields a significant computational overhead, hampers widespread usage and results in the predicted brain-age to be sensitive to preprocessing parameters. Here we describe a 3D Convolutional Neural Network (CNN) based on the ResNet architecture being trained on raw, non-registered T$_ 1$-weighted MRI data of N=10,691 samples from the German National Cohort and additionally applied and validated in N=2,173 samples from three independent studies using transfer learning. For comparison, state-of-the-art models using preprocessed neuroimaging data are trained and validated on the same samples. The 3D CNN using raw neuroimaging data predicts age with a mean average deviation of 2.84 years, outperforming the state-of-the-art brain-age models using preprocessed data. Since our approach is invariant to preprocessing software and parameter choices, it enables faster, more robust and more accurate brain-age modeling.
The PHOTON Wizard -- Towards Educational Machine Learning Code Generators
Feb 13, 2020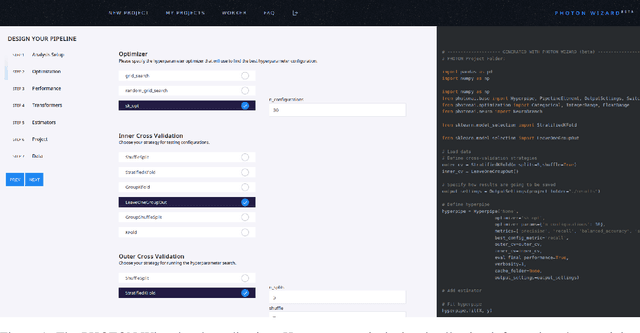
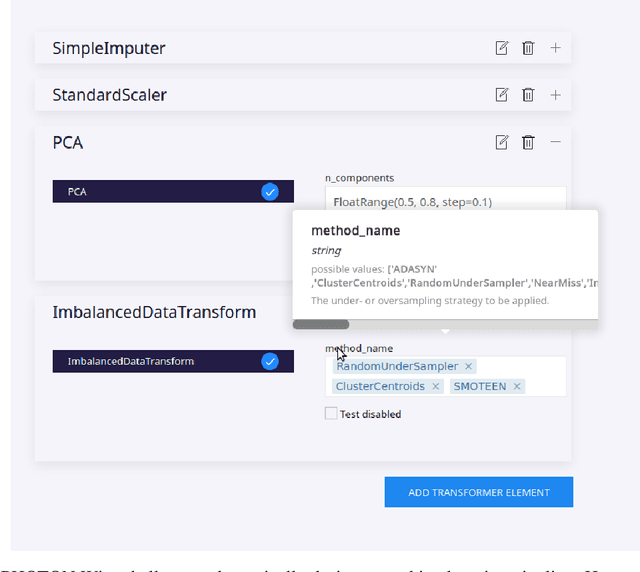
Abstract:Despite the tremendous efforts to democratize machine learning, especially in applied-science, the application is still often hampered by the lack of coding skills. As we consider programmatic understanding key to building effective and efficient machine learning solutions, we argue for a novel educational approach that builds upon the accessibility and acceptance of graphical user interfaces to convey programming skills to an applied-science target group. We outline a proof-of-concept, open-source web application, the PHOTON Wizard, which dynamically translates GUI interactions into valid source code for the Python machine learning framework PHOTON. Thereby, users possessing theoretical machine learning knowledge gain key insights into the model development workflow as well as an intuitive understanding of custom implementations. Specifically, the PHOTON Wizard integrates the concept of Educational Machine Learning Code Generators to teach users how to write code for designing, training, optimizing and evaluating custom machine learning pipelines.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge