Alireza Rahimi
Heart2Mind: Human-Centered Contestable Psychiatric Disorder Diagnosis System using Wearable ECG Monitors
May 16, 2025



Abstract:Psychiatric disorders affect millions globally, yet their diagnosis faces significant challenges in clinical practice due to subjective assessments and accessibility concerns, leading to potential delays in treatment. To help address this issue, we present Heart2Mind, a human-centered contestable psychiatric disorder diagnosis system using wearable electrocardiogram (ECG) monitors. Our approach leverages cardiac biomarkers, particularly heart rate variability (HRV) and R-R intervals (RRI) time series, as objective indicators of autonomic dysfunction in psychiatric conditions. The system comprises three key components: (1) a Cardiac Monitoring Interface (CMI) for real-time data acquisition from Polar H9/H10 devices; (2) a Multi-Scale Temporal-Frequency Transformer (MSTFT) that processes RRI time series through integrated time-frequency domain analysis; (3) a Contestable Diagnosis Interface (CDI) combining Self-Adversarial Explanations (SAEs) with contestable Large Language Models (LLMs). Our MSTFT achieves 91.7% accuracy on the HRV-ACC dataset using leave-one-out cross-validation, outperforming state-of-the-art methods. SAEs successfully detect inconsistencies in model predictions by comparing attention-based and gradient-based explanations, while LLMs enable clinicians to validate correct predictions and contest erroneous ones. This work demonstrates the feasibility of combining wearable technology with Explainable Artificial Intelligence (XAI) and contestable LLMs to create a transparent, contestable system for psychiatric diagnosis that maintains clinical oversight while leveraging advanced AI capabilities. Our implementation is publicly available at: https://github.com/Analytics-Everywhere-Lab/heart2mind.
Achieving Pareto Optimality using Efficient Parameter Reduction for DNNs in Resource-Constrained Edge Environment
Mar 14, 2024Abstract:This paper proposes an optimization of an existing Deep Neural Network (DNN) that improves its hardware utilization and facilitates on-device training for resource-constrained edge environments. We implement efficient parameter reduction strategies on Xception that shrink the model size without sacrificing accuracy, thus decreasing memory utilization during training. We evaluate our model in two experiments: Caltech-101 image classification and PCB defect detection and compare its performance against the original Xception and lightweight models, EfficientNetV2B1 and MobileNetV2. The results of the Caltech-101 image classification show that our model has a better test accuracy (76.21%) than Xception (75.89%), uses less memory on average (847.9MB) than Xception (874.6MB), and has faster training and inference times. The lightweight models overfit with EfficientNetV2B1 having a 30.52% test accuracy and MobileNetV2 having a 58.11% test accuracy. Both lightweight models have better memory usage than our model and Xception. On the PCB defect detection, our model has the best test accuracy (90.30%), compared to Xception (88.10%), EfficientNetV2B1 (55.25%), and MobileNetV2 (50.50%). MobileNetV2 has the least average memory usage (849.4MB), followed by our model (865.8MB), then EfficientNetV2B1 (874.8MB), and Xception has the highest (893.6MB). We further experiment with pre-trained weights and observe that memory usage decreases thereby showing the benefits of transfer learning. A Pareto analysis of the models' performance shows that our optimized model architecture satisfies accuracy and low memory utilization objectives.
Automatic Diagnosis of COVID-19 from CT Images using CycleGAN and Transfer Learning
Apr 24, 2021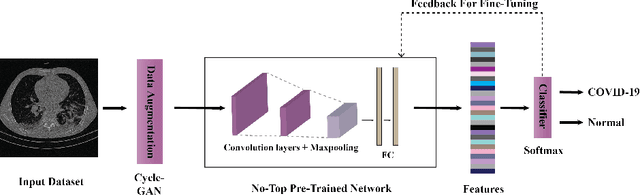
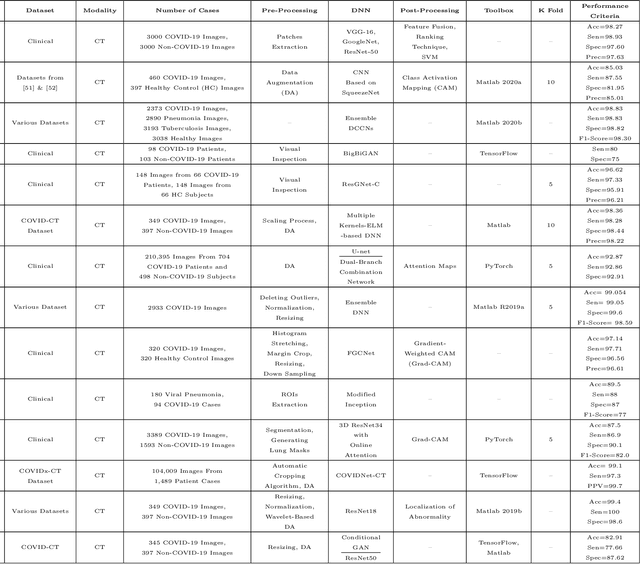
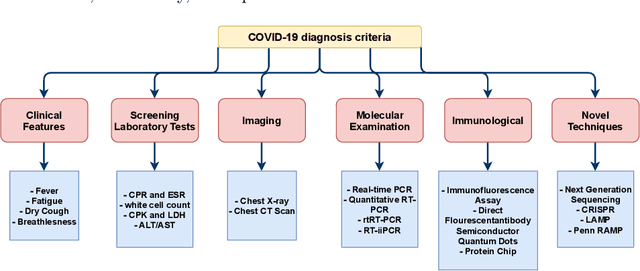
Abstract:The outbreak of the corona virus disease (COVID-19) has changed the lives of most people on Earth. Given the high prevalence of this disease, its correct diagnosis in order to quarantine patients is of the utmost importance in steps of fighting this pandemic. Among the various modalities used for diagnosis, medical imaging, especially computed tomography (CT) imaging, has been the focus of many previous studies due to its accuracy and availability. In addition, automation of diagnostic methods can be of great help to physicians. In this paper, a method based on pre-trained deep neural networks is presented, which, by taking advantage of a cyclic generative adversarial net (CycleGAN) model for data augmentation, has reached state-of-the-art performance for the task at hand, i.e., 99.60% accuracy. Also, in order to evaluate the method, a dataset containing 3163 images from 189 patients has been collected and labeled by physicians. Unlike prior datasets, normal data have been collected from people suspected of having COVID-19 disease and not from data from other diseases, and this database is made available publicly.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge