Robert Timmerman
Leveraging Support Vector Regression for Outcome Prediction in Personalized Ultra-fractionated Stereotactic Adaptive Radiotherapy
Sep 09, 2025Abstract:Personalized ultra-fractionated stereotactic adaptive radiotherapy (PULSAR) is a novel treatment that delivers radiation in pulses of protracted intervals. Accurate prediction of gross tumor volume (GTV) changes through regression models has substantial prognostic value. This study aims to develop a multi-omics based support vector regression (SVR) model for predicting GTV change. A retrospective cohort of 39 patients with 69 brain metastases was analyzed, based on radiomics (MRI images) and dosiomics (dose maps) features. Delta features were computed to capture relative changes between two time points. A feature selection pipeline using least absolute shrinkage and selection operator (Lasso) algorithm with weight- or frequency-based ranking criterion was implemented. SVR models with various kernels were evaluated using the coefficient of determination (R2) and relative root mean square error (RRMSE). Five-fold cross-validation with 10 repeats was employed to mitigate the limitation of small data size. Multi-omics models that integrate radiomics, dosiomics, and their delta counterparts outperform individual-omics models. Delta-radiomic features play a critical role in enhancing prediction accuracy relative to features at single time points. The top-performing model achieves an R2 of 0.743 and an RRMSE of 0.022. The proposed multi-omics SVR model shows promising performance in predicting continuous change of GTV. It provides a more quantitative and personalized approach to assist patient selection and treatment adjustment in PULSAR.
Exploring Strategies for Personalized Radiation Therapy: Part III Identifying genetic determinants for Radiation Response with Meta Learning
Aug 11, 2025



Abstract:Radiation response in cancer is shaped by complex, patient specific biology, yet current treatment strategies often rely on uniform dose prescriptions without accounting for tumor heterogeneity. In this study, we introduce a meta learning framework for one-shot prediction of radiosensitivity measured by SF2 using cell line level gene expression data. Unlike the widely used Radiosensitivity Index RSI a rank-based linear model trained on a fixed 10-gene signature, our proposed meta-learned model allows the importance of each gene to vary by sample through fine tuning. This flexibility addresses key limitations of static models like RSI, which assume uniform gene contributions across tumor types and discard expression magnitude and gene gene interactions. Our results show that meta learning offers robust generalization to unseen samples and performs well in tumor subgroups with high radiosensitivity variability, such as adenocarcinoma and large cell carcinoma. By learning transferable structure across tasks while preserving sample specific adaptability, our approach enables rapid adaptation to individual samples, improving predictive accuracy across diverse tumor subtypes while uncovering context dependent patterns of gene influence that may inform personalized therapy.
Understanding the PULSAR Effect in Combined Radiotherapy and Immunotherapy through Attention Mechanisms with a Transformer Model
Mar 07, 2024Abstract:PULSAR (personalized, ultra-fractionated stereotactic adaptive radiotherapy) is the adaptation of stereotactic ablative radiotherapy towards personalized cancer management. For the first time, we applied a transformer-based attention mechanism to investigate the underlying interactions between combined PULSAR and PD-L1 blockade immunotherapy based on a murine cancer model (Lewis Lung Carcinoma, LLC). The proposed approach is able to predict the trend of tumor volume change semi-quantitatively, and excels in identifying the potential causal relationships through both self-attention and cross-attention scores.
Performance Deterioration of Deep Learning Models after Clinical Deployment: A Case Study with Auto-segmentation for Definitive Prostate Cancer Radiotherapy
Oct 11, 2022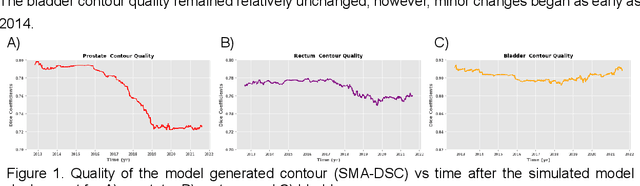
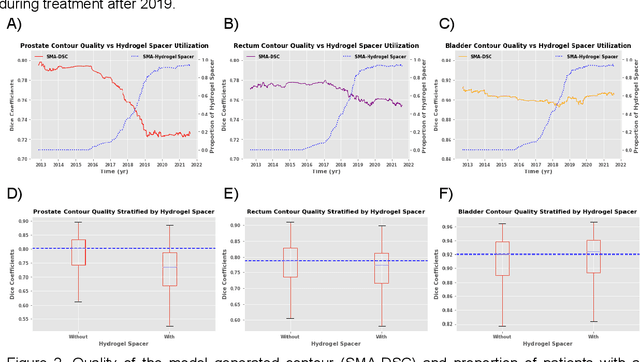
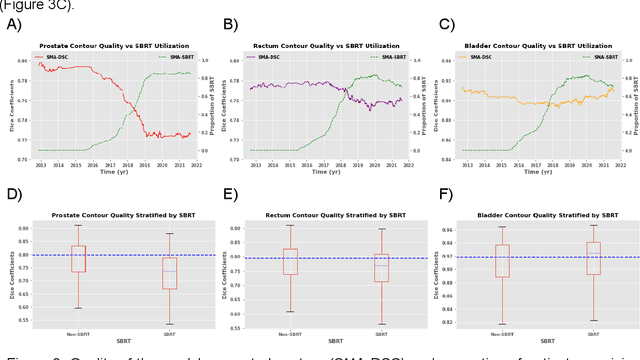
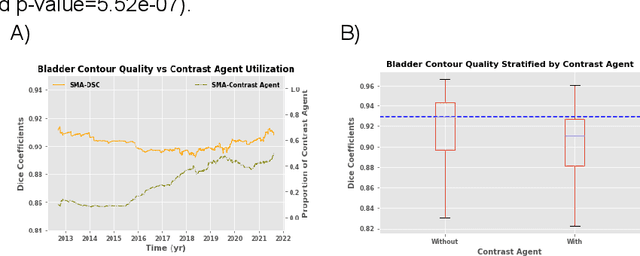
Abstract:In the past decade, deep learning (DL)-based artificial intelligence (AI) has witnessed unprecedented success and has led to much excitement in medicine. However, many successful models have not been implemented in the clinic predominantly due to concerns regarding the lack of interpretability and generalizability in both spatial and temporal domains. In this work, we used a DL-based auto segmentation model for intact prostate patients to observe any temporal performance changes and then correlate them to possible explanatory variables. We retrospectively simulated the clinical implementation of our DL model to investigate temporal performance trends. Our cohort included 912 patients with prostate cancer treated with definitive radiotherapy from January 2006 to August 2021 at the University of Texas Southwestern Medical Center (UTSW). We trained a U-Net-based DL auto segmentation model on the data collected before 2012 and tested it on data collected from 2012 to 2021 to simulate the clinical deployment of the trained model starting in 2012. We visualize the trends using a simple moving average curve and used ANOVA and t-test to investigate the impact of various clinical factors. The prostate and rectum contour quality decreased rapidly after 2016-2017. Stereotactic body radiotherapy (SBRT) and hydrogel spacer use were significantly associated with prostate contour quality (p=5.6e-12 and 0.002, respectively). SBRT and physicians' styles are significantly associated with the rectum contour quality (p=0.0005 and 0.02, respectively). Only the presence of contrast within the bladder significantly affected the bladder contour quality (p=1.6e-7). We showed that DL model performance decreased over time in concordance with changes in clinical practice patterns and changes in clinical personnel.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge