Martin Bishop
Physics-Informed Neural Operators for Cardiac Electrophysiology
Nov 11, 2025Abstract:Accurately simulating systems governed by PDEs, such as voltage fields in cardiac electrophysiology (EP) modelling, remains a significant modelling challenge. Traditional numerical solvers are computationally expensive and sensitive to discretisation, while canonical deep learning methods are data-hungry and struggle with chaotic dynamics and long-term predictions. Physics-Informed Neural Networks (PINNs) mitigate some of these issues by incorporating physical constraints in the learning process, yet they remain limited by mesh resolution and long-term predictive stability. In this work, we propose a Physics-Informed Neural Operator (PINO) approach to solve PDE problems in cardiac EP. Unlike PINNs, PINO models learn mappings between function spaces, allowing them to generalise to multiple mesh resolutions and initial conditions. Our results show that PINO models can accurately reproduce cardiac EP dynamics over extended time horizons and across multiple propagation scenarios, including zero-shot evaluations on scenarios unseen during training. Additionally, our PINO models maintain high predictive quality in long roll-outs (where predictions are recursively fed back as inputs), and can scale their predictive resolution by up to 10x the training resolution. These advantages come with a significant reduction in simulation time compared to numerical PDE solvers, highlighting the potential of PINO-based approaches for efficient and scalable cardiac EP simulations.
Cardiac Digital Twins at Scale from MRI: Open Tools and Representative Models from ~55000 UK Biobank Participants
May 27, 2025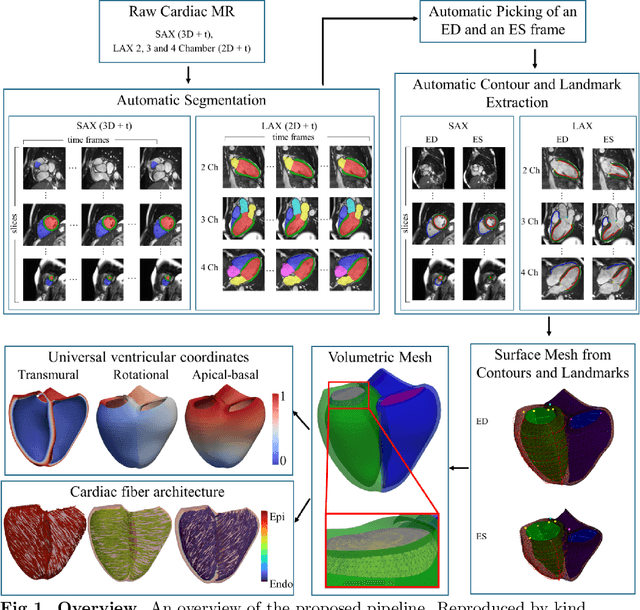
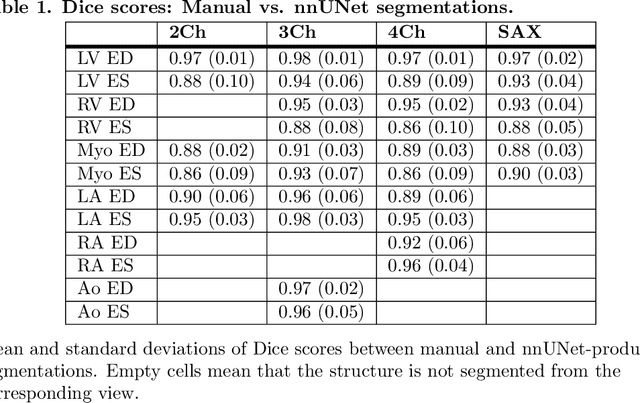
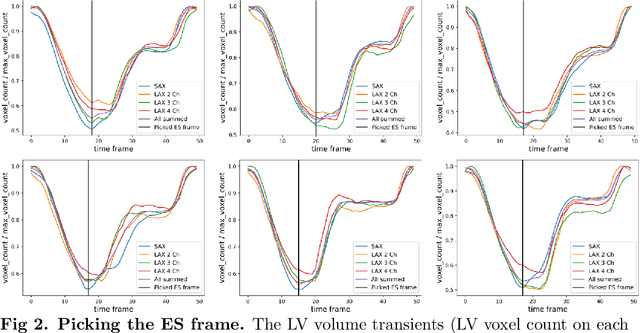
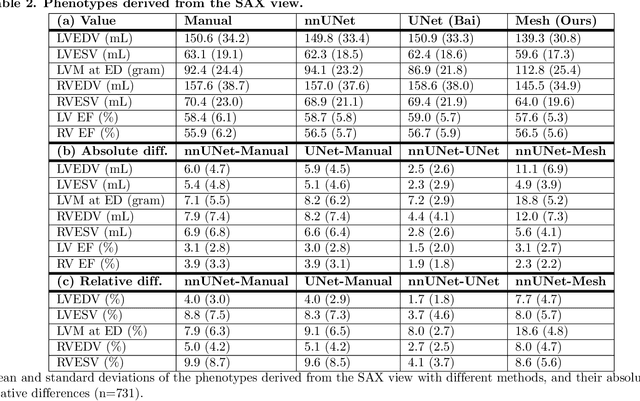
Abstract:A cardiac digital twin is a virtual replica of a patient's heart for screening, diagnosis, prognosis, risk assessment, and treatment planning of cardiovascular diseases. This requires an anatomically accurate patient-specific 3D structural representation of the heart, suitable for electro-mechanical simulations or study of disease mechanisms. However, generation of cardiac digital twins at scale is demanding and there are no public repositories of models across demographic groups. We describe an automatic open-source pipeline for creating patient-specific left and right ventricular meshes from cardiovascular magnetic resonance images, its application to a large cohort of ~55000 participants from UK Biobank, and the construction of the most comprehensive cohort of adult heart models to date, comprising 1423 representative meshes across sex (male, female), body mass index (range: 16 - 42 kg/m$^2$) and age (range: 49 - 80 years). Our code is available at https://github.com/cdttk/biv-volumetric-meshing/tree/plos2025 , and pre-trained networks, representative volumetric meshes with fibers and UVCs will be made available soon.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge