Laura Marcu
Pixel Super-Resolved Fluorescence Lifetime Imaging Using Deep Learning
Dec 18, 2025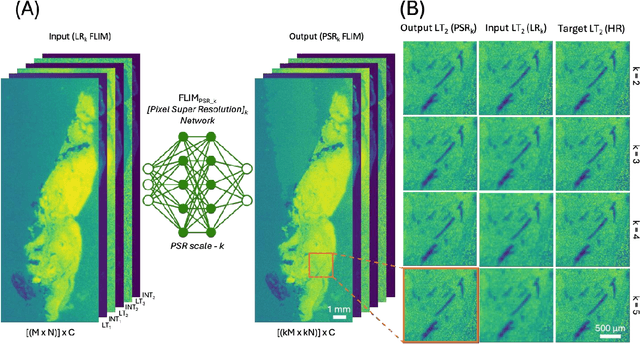
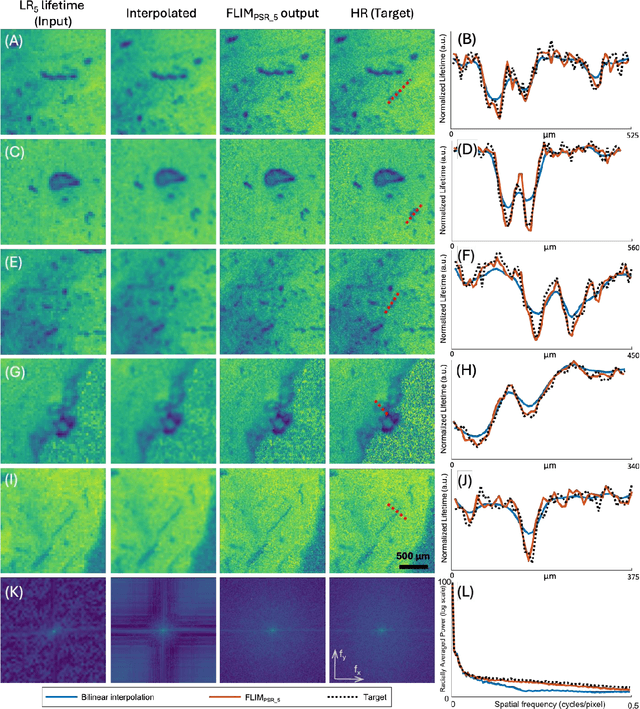
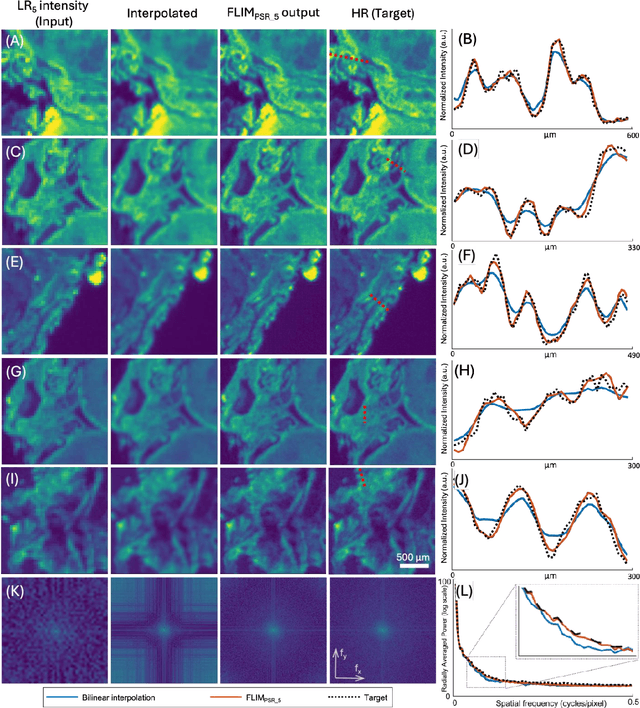
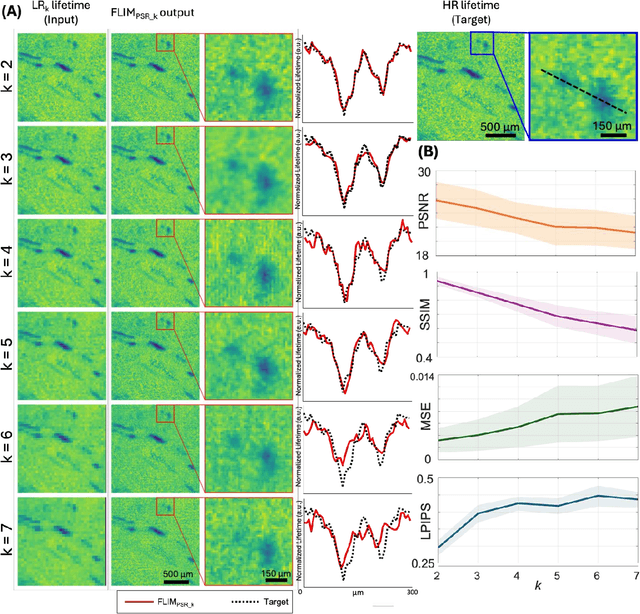
Abstract:Fluorescence lifetime imaging microscopy (FLIM) is a powerful quantitative technique that provides metabolic and molecular contrast, offering strong translational potential for label-free, real-time diagnostics. However, its clinical adoption remains limited by long pixel dwell times and low signal-to-noise ratio (SNR), which impose a stricter resolution-speed trade-off than conventional optical imaging approaches. Here, we introduce FLIM_PSR_k, a deep learning-based multi-channel pixel super-resolution (PSR) framework that reconstructs high-resolution FLIM images from data acquired with up to a 5-fold increased pixel size. The model is trained using the conditional generative adversarial network (cGAN) framework, which, compared to diffusion model-based alternatives, delivers a more robust PSR reconstruction with substantially shorter inference times, a crucial advantage for practical deployment. FLIM_PSR_k not only enables faster image acquisition but can also alleviate SNR limitations in autofluorescence-based FLIM. Blind testing on held-out patient-derived tumor tissue samples demonstrates that FLIM_PSR_k reliably achieves a super-resolution factor of k = 5, resulting in a 25-fold increase in the space-bandwidth product of the output images and revealing fine architectural features lost in lower-resolution inputs, with statistically significant improvements across various image quality metrics. By increasing FLIM's effective spatial resolution, FLIM_PSR_k advances lifetime imaging toward faster, higher-resolution, and hardware-flexible implementations compatible with low-numerical-aperture and miniaturized platforms, better positioning FLIM for translational applications.
Data-Centric Learning Framework for Real-Time Detection of Aiming Beam in Fluorescence Lifetime Imaging Guided Surgery
Nov 11, 2024



Abstract:This study introduces a novel data-centric approach to improve real-time surgical guidance using fiber-based fluorescence lifetime imaging (FLIm). A key aspect of the methodology is the accurate detection of the aiming beam, which is essential for localizing points used to map FLIm measurements onto the tissue region within the surgical field. The primary challenge arises from the complex and variable conditions encountered in the surgical environment, particularly in Transoral Robotic Surgery (TORS). Uneven illumination in the surgical field can cause reflections, reduce contrast, and results in inconsistent color representation, further complicating aiming beam detection. To overcome these challenges, an instance segmentation model was developed using a data-centric training strategy that improves accuracy by minimizing label noise and enhancing detection robustness. The model was evaluated on a dataset comprising 40 in vivo surgical videos, demonstrating a median detection rate of 85%. This performance was maintained when the model was integrated in a clinical system, achieving a similar detection rate of 85% during TORS procedures conducted in patients. The system's computational efficiency, measured at approximately 24 frames per second (FPS), was sufficient for real-time surgical guidance. This study enhances the reliability of FLIm-based aiming beam detection in complex surgical environments, advancing the feasibility of real-time, image-guided interventions for improved surgical precision
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge