John G. Golfinos
Repurposing the scientific literature with vision-language models
Feb 26, 2025Abstract:Research in AI for Science often focuses on using AI technologies to augment components of the scientific process, or in some cases, the entire scientific method; how about AI for scientific publications? Peer-reviewed journals are foundational repositories of specialized knowledge, written in discipline-specific language that differs from general Internet content used to train most large language models (LLMs) and vision-language models (VLMs). We hypothesized that by combining a family of scientific journals with generative AI models, we could invent novel tools for scientific communication, education, and clinical care. We converted 23,000 articles from Neurosurgery Publications into a multimodal database - NeuroPubs - of 134 million words and 78,000 image-caption pairs to develop six datasets for building AI models. We showed that the content of NeuroPubs uniquely represents neurosurgery-specific clinical contexts compared with broader datasets and PubMed. For publishing, we employed generalist VLMs to automatically generate graphical abstracts from articles. Editorial board members rated 70% of these as ready for publication without further edits. For education, we generated 89,587 test questions in the style of the ABNS written board exam, which trainee and faculty neurosurgeons found indistinguishable from genuine examples 54% of the time. We used these questions alongside a curriculum learning process to track knowledge acquisition while training our 34 billion-parameter VLM (CNS-Obsidian). In a blinded, randomized controlled trial, we demonstrated the non-inferiority of CNS-Obsidian to GPT-4o (p = 0.1154) as a diagnostic copilot for a neurosurgical service. Our findings lay a novel foundation for AI with Science and establish a framework to elevate scientific communication using state-of-the-art generative artificial intelligence while maintaining rigorous quality standards.
Artificial-intelligence-based molecular classification of diffuse gliomas using rapid, label-free optical imaging
Mar 23, 2023Abstract:Molecular classification has transformed the management of brain tumors by enabling more accurate prognostication and personalized treatment. However, timely molecular diagnostic testing for patients with brain tumors is limited, complicating surgical and adjuvant treatment and obstructing clinical trial enrollment. In this study, we developed DeepGlioma, a rapid ($< 90$ seconds), artificial-intelligence-based diagnostic screening system to streamline the molecular diagnosis of diffuse gliomas. DeepGlioma is trained using a multimodal dataset that includes stimulated Raman histology (SRH); a rapid, label-free, non-consumptive, optical imaging method; and large-scale, public genomic data. In a prospective, multicenter, international testing cohort of patients with diffuse glioma ($n=153$) who underwent real-time SRH imaging, we demonstrate that DeepGlioma can predict the molecular alterations used by the World Health Organization to define the adult-type diffuse glioma taxonomy (IDH mutation, 1p19q co-deletion and ATRX mutation), achieving a mean molecular classification accuracy of $93.3\pm 1.6\%$. Our results represent how artificial intelligence and optical histology can be used to provide a rapid and scalable adjunct to wet lab methods for the molecular screening of patients with diffuse glioma.
Contrastive Representation Learning for Rapid Intraoperative Diagnosis of Skull Base Tumors Imaged Using Stimulated Raman Histology
Aug 08, 2021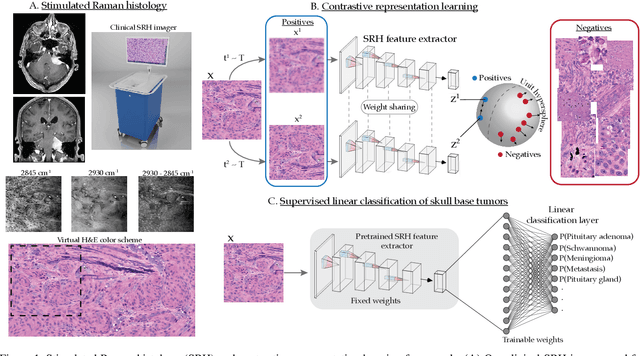
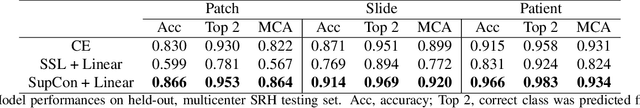
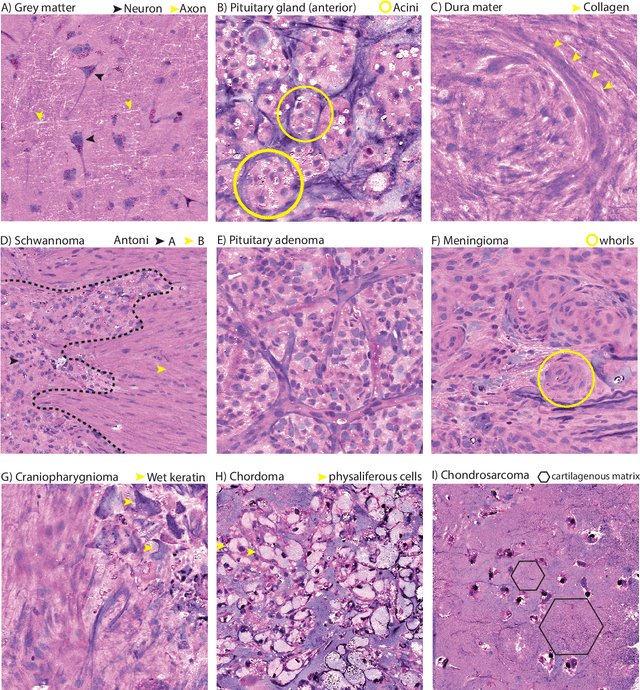
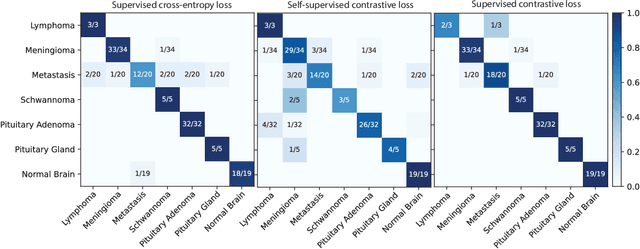
Abstract:Background: Accurate diagnosis of skull base tumors is essential for providing personalized surgical treatment strategies. Intraoperative diagnosis can be challenging due to tumor diversity and lack of intraoperative pathology resources. Objective: To develop an independent and parallel intraoperative pathology workflow that can provide rapid and accurate skull base tumor diagnoses using label-free optical imaging and artificial intelligence (AI). Method: We used a fiber laser-based, label-free, non-consumptive, high-resolution microscopy method ($<$ 60 sec per 1 $\times$ 1 mm$^\text{2}$), called stimulated Raman histology (SRH), to image a consecutive, multicenter cohort of skull base tumor patients. SRH images were then used to train a convolutional neural network (CNN) model using three representation learning strategies: cross-entropy, self-supervised contrastive learning, and supervised contrastive learning. Our trained CNN models were tested on a held-out, multicenter SRH dataset. Results: SRH was able to image the diagnostic features of both benign and malignant skull base tumors. Of the three representation learning strategies, supervised contrastive learning most effectively learned the distinctive and diagnostic SRH image features for each of the skull base tumor types. In our multicenter testing set, cross-entropy achieved an overall diagnostic accuracy of 91.5%, self-supervised contrastive learning 83.9%, and supervised contrastive learning 96.6%. Our trained model was able to identify tumor-normal margins and detect regions of microscopic tumor infiltration in whole-slide SRH images. Conclusion: SRH with AI models trained using contrastive representation learning can provide rapid and accurate intraoperative diagnosis of skull base tumors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge