Jerry L. Prince
HACA3: A Unified Approach for Multi-site MR Image Harmonization
Dec 12, 2022Abstract:The lack of standardization is a prominent issue in magnetic resonance (MR) imaging. This often causes undesired contrast variations due to differences in hardware and acquisition parameters. In recent years, MR harmonization using image synthesis with disentanglement has been proposed to compensate for the undesired contrast variations. Despite the success of existing methods, we argue that three major improvements can be made. First, most existing methods are built upon the assumption that multi-contrast MR images of the same subject share the same anatomy. This assumption is questionable since different MR contrasts are specialized to highlight different anatomical features. Second, these methods often require a fixed set of MR contrasts for training (e.g., both Tw-weighted and T2-weighted images must be available), which limits their applicability. Third, existing methods generally are sensitive to imaging artifacts. In this paper, we present a novel approach, Harmonization with Attention-based Contrast, Anatomy, and Artifact Awareness (HACA3), to address these three issues. We first propose an anatomy fusion module that enables HACA3 to respect the anatomical differences between MR contrasts. HACA3 is also robust to imaging artifacts and can be trained and applied to any set of MR contrasts. Experiments show that HACA3 achieves state-of-the-art performance under multiple image quality metrics. We also demonstrate the applicability of HACA3 on downstream tasks with diverse MR datasets acquired from 21 sites with different field strengths, scanner platforms, and acquisition protocols.
Deep filter bank regression for super-resolution of anisotropic MR brain images
Sep 06, 2022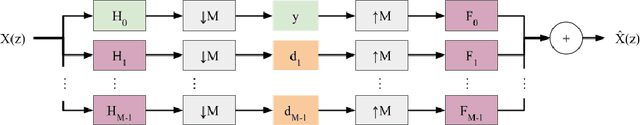
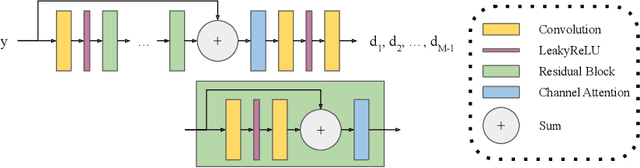
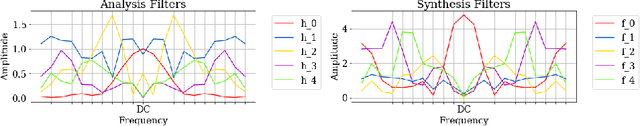
Abstract:In 2D multi-slice magnetic resonance (MR) acquisition, the through-plane signals are typically of lower resolution than the in-plane signals. While contemporary super-resolution (SR) methods aim to recover the underlying high-resolution volume, the estimated high-frequency information is implicit via end-to-end data-driven training rather than being explicitly stated and sought. To address this, we reframe the SR problem statement in terms of perfect reconstruction filter banks, enabling us to identify and directly estimate the missing information. In this work, we propose a two-stage approach to approximate the completion of a perfect reconstruction filter bank corresponding to the anisotropic acquisition of a particular scan. In stage 1, we estimate the missing filters using gradient descent and in stage 2, we use deep networks to learn the mapping from coarse coefficients to detail coefficients. In addition, the proposed formulation does not rely on external training data, circumventing the need for domain shift correction. Under our approach, SR performance is improved particularly in "slice gap" scenarios, likely due to the constrained solution space imposed by the framework.
Tagged-MRI Sequence to Audio Synthesis via Self Residual Attention Guided Heterogeneous Translator
Jun 09, 2022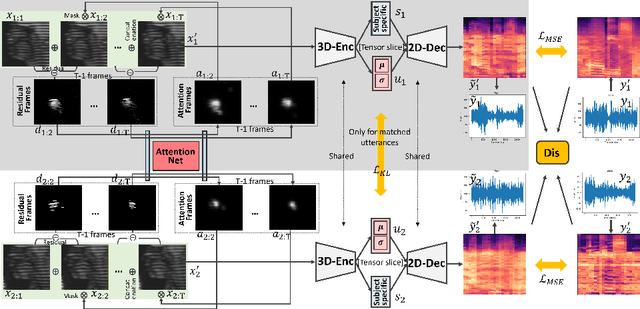
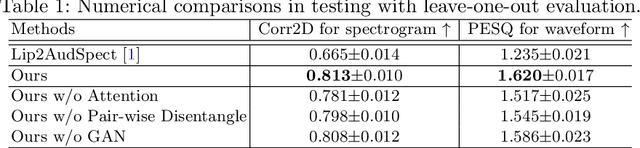
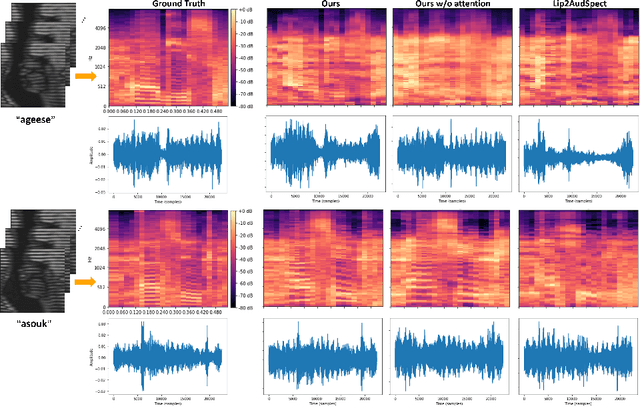
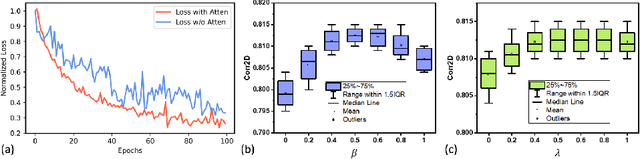
Abstract:Understanding the underlying relationship between tongue and oropharyngeal muscle deformation seen in tagged-MRI and intelligible speech plays an important role in advancing speech motor control theories and treatment of speech related-disorders. Because of their heterogeneous representations, however, direct mapping between the two modalities -- i.e., two-dimensional (mid-sagittal slice) plus time tagged-MRI sequence and its corresponding one-dimensional waveform -- is not straightforward. Instead, we resort to two-dimensional spectrograms as an intermediate representation, which contains both pitch and resonance, from which to develop an end-to-end deep learning framework to translate from a sequence of tagged-MRI to its corresponding audio waveform with limited dataset size.~Our framework is based on a novel fully convolutional asymmetry translator with guidance of a self residual attention strategy to specifically exploit the moving muscular structures during speech.~In addition, we leverage a pairwise correlation of the samples with the same utterances with a latent space representation disentanglement strategy.~Furthermore, we incorporate an adversarial training approach with generative adversarial networks to offer improved realism on our generated spectrograms.~Our experimental results, carried out with a total of 63 tagged-MRI sequences alongside speech acoustics, showed that our framework enabled the generation of clear audio waveforms from a sequence of tagged-MRI, surpassing competing methods. Thus, our framework provides the great potential to help better understand the relationship between the two modalities.
Disentangling A Single MR Modality
May 10, 2022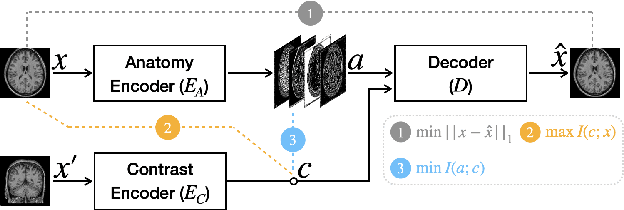
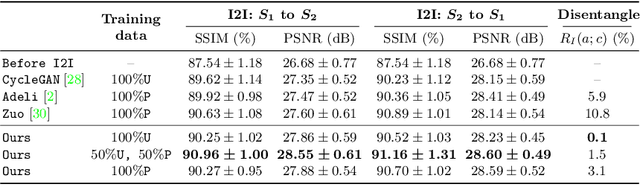
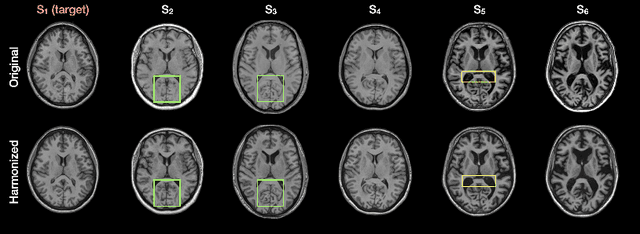
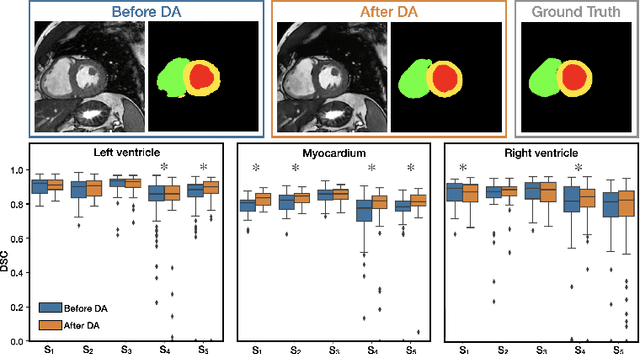
Abstract:Disentangling anatomical and contrast information from medical images has gained attention recently, demonstrating benefits for various image analysis tasks. Current methods learn disentangled representations using either paired multi-modal images with the same underlying anatomy or auxiliary labels (e.g., manual delineations) to provide inductive bias for disentanglement. However, these requirements could significantly increase the time and cost in data collection and limit the applicability of these methods when such data are not available. Moreover, these methods generally do not guarantee disentanglement. In this paper, we present a novel framework that learns theoretically and practically superior disentanglement from single modality magnetic resonance images. Moreover, we propose a new information-based metric to quantitatively evaluate disentanglement. Comparisons over existing disentangling methods demonstrate that the proposed method achieves superior performance in both disentanglement and cross-domain image-to-image translation tasks.
Coordinate Translator for Learning Deformable Medical Image Registration
Mar 05, 2022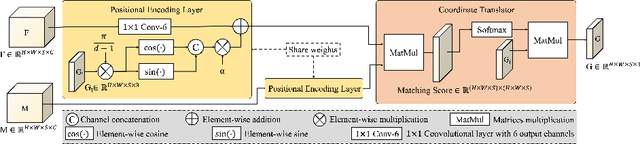
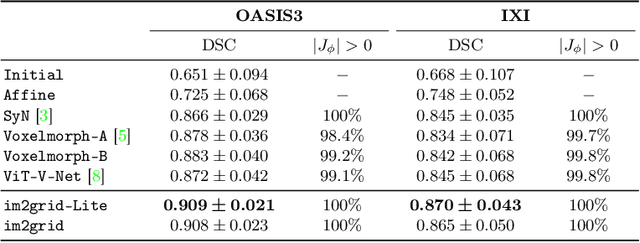
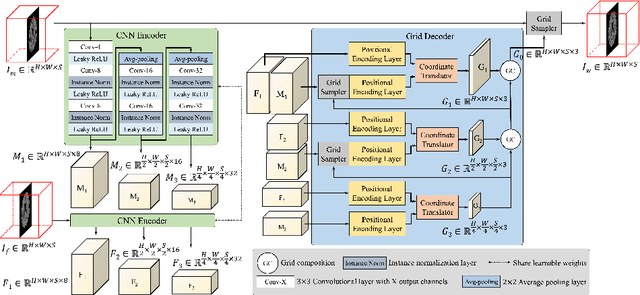
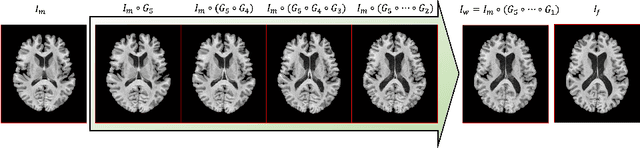
Abstract:The majority of deep learning (DL) based deformable image registration methods use convolutional neural networks (CNNs) to estimate displacement fields from pairs of moving and fixed images. This, however, requires the convolutional kernels in the CNN to not only extract intensity features from the inputs but also understand image coordinate systems. We argue that the latter task is challenging for traditional CNNs, limiting their performance in registration tasks. To tackle this problem, we first introduce Coordinate Translator (CoTr), a differentiable module that identifies matched features between the fixed and moving image and outputs their coordinate correspondences without the need for training. It unloads the burden of understanding image coordinate systems for CNNs, allowing them to focus on feature extraction. We then propose a novel deformable registration network, im2grid, that uses multiple CoTr's with the hierarchical features extracted from a CNN encoder and outputs a deformation field in a coarse-to-fine fashion. We compared im2grid with the state-of-the-art DL and non-DL methods for unsupervised 3D magnetic resonance image registration. Our experiments show that im2grid outperforms these methods both qualitatively and quantitatively.
Structure-aware Unsupervised Tagged-to-Cine MRI Synthesis with Self Disentanglement
Feb 25, 2022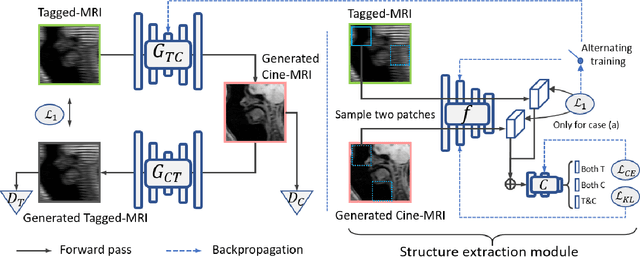

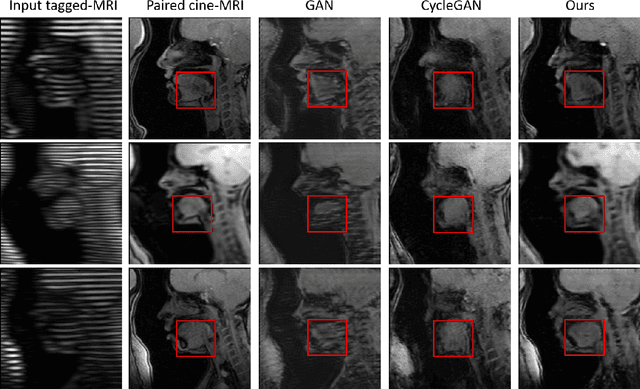
Abstract:Cycle reconstruction regularized adversarial training -- e.g., CycleGAN, DiscoGAN, and DualGAN -- has been widely used for image style transfer with unpaired training data. Several recent works, however, have shown that local distortions are frequent, and structural consistency cannot be guaranteed. Targeting this issue, prior works usually relied on additional segmentation or consistent feature extraction steps that are task-specific. To counter this, this work aims to learn a general add-on structural feature extractor, by explicitly enforcing the structural alignment between an input and its synthesized image. Specifically, we propose a novel input-output image patches self-training scheme to achieve a disentanglement of underlying anatomical structures and imaging modalities. The translator and structure encoder are updated, following an alternating training protocol. In addition, the information w.r.t. imaging modality can be eliminated with an asymmetric adversarial game. We train, validate, and test our network on 1,768, 416, and 1,560 unpaired subject-independent slices of tagged and cine magnetic resonance imaging from a total of twenty healthy subjects, respectively, demonstrating superior performance over competing methods.
Generative Self-training for Cross-domain Unsupervised Tagged-to-Cine MRI Synthesis
Jun 23, 2021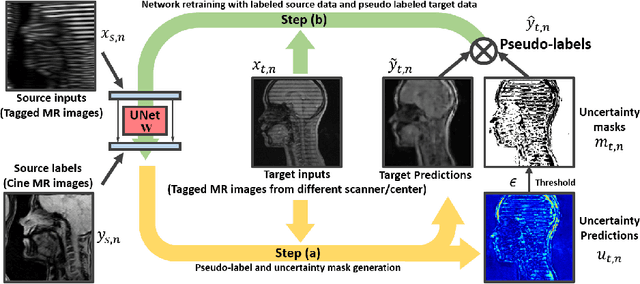
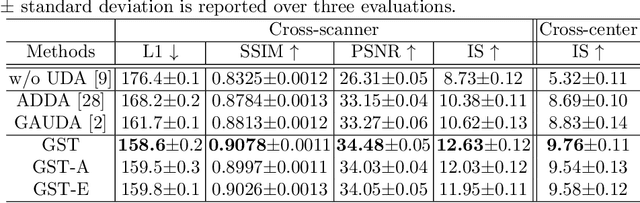
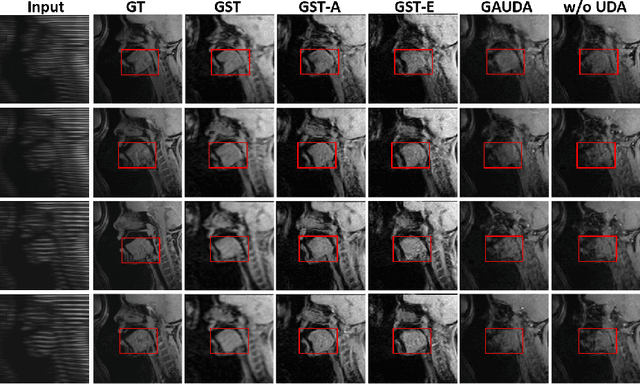
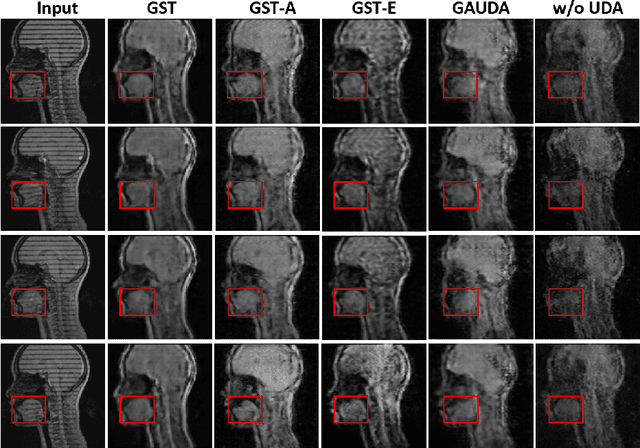
Abstract:Self-training based unsupervised domain adaptation (UDA) has shown great potential to address the problem of domain shift, when applying a trained deep learning model in a source domain to unlabeled target domains. However, while the self-training UDA has demonstrated its effectiveness on discriminative tasks, such as classification and segmentation, via the reliable pseudo-label selection based on the softmax discrete histogram, the self-training UDA for generative tasks, such as image synthesis, is not fully investigated. In this work, we propose a novel generative self-training (GST) UDA framework with continuous value prediction and regression objective for cross-domain image synthesis. Specifically, we propose to filter the pseudo-label with an uncertainty mask, and quantify the predictive confidence of generated images with practical variational Bayes learning. The fast test-time adaptation is achieved by a round-based alternative optimization scheme. We validated our framework on the tagged-to-cine magnetic resonance imaging (MRI) synthesis problem, where datasets in the source and target domains were acquired from different scanners or centers. Extensive validations were carried out to verify our framework against popular adversarial training UDA methods. Results show that our GST, with tagged MRI of test subjects in new target domains, improved the synthesis quality by a large margin, compared with the adversarial training UDA methods.
MR Slice Profile Estimation by Learning to Match Internal Patch Distributions
Mar 31, 2021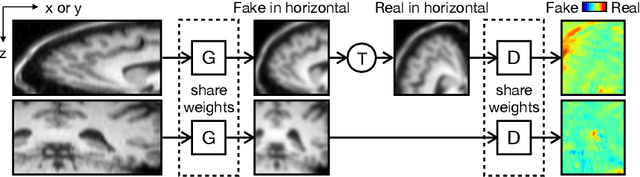
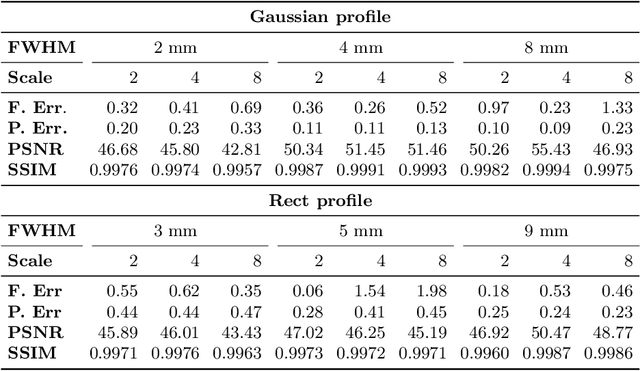
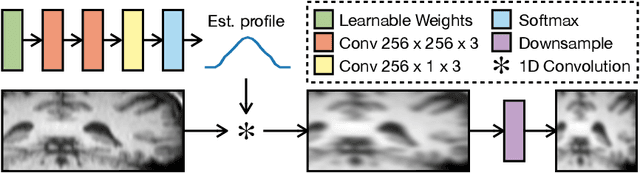
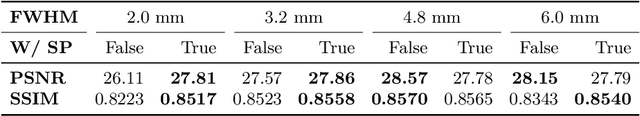
Abstract:To super-resolve the through-plane direction of a multi-slice 2D magnetic resonance (MR) image, its slice selection profile can be used as the degeneration model from high resolution (HR) to low resolution (LR) to create paired data when training a supervised algorithm. Existing super-resolution algorithms make assumptions about the slice selection profile since it is not readily known for a given image. In this work, we estimate a slice selection profile given a specific image by learning to match its internal patch distributions. Specifically, we assume that after applying the correct slice selection profile, the image patch distribution along HR in-plane directions should match the distribution along the LR through-plane direction. Therefore, we incorporate the estimation of a slice selection profile as part of learning a generator in a generative adversarial network (GAN). In this way, the slice selection profile can be learned without any external data. Our algorithm was tested using simulations from isotropic MR images, incorporated in a through-plane super-resolution algorithm to demonstrate its benefits, and also used as a tool to measure image resolution. Our code is at https://github.com/shuohan/espreso2.
Information-based Disentangled Representation Learning for Unsupervised MR Harmonization
Mar 24, 2021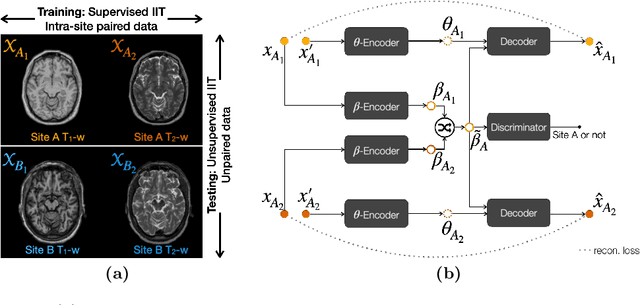
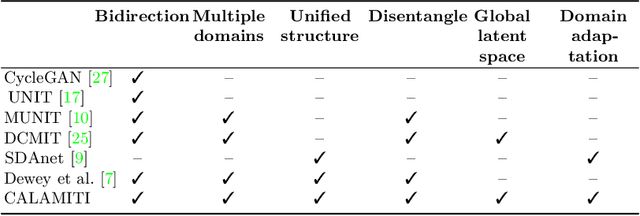
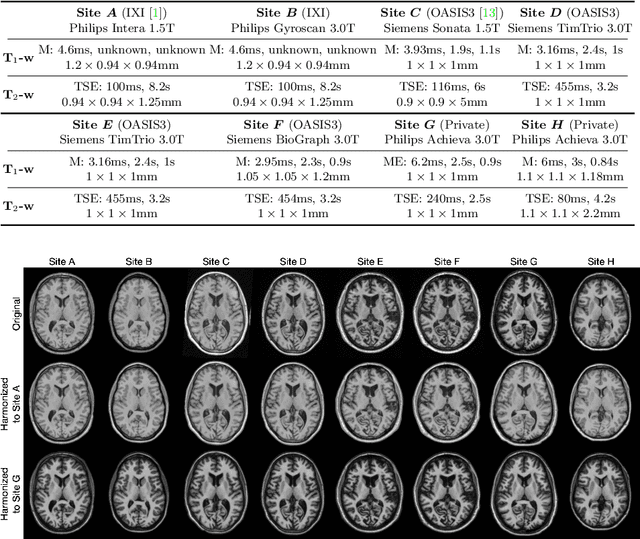
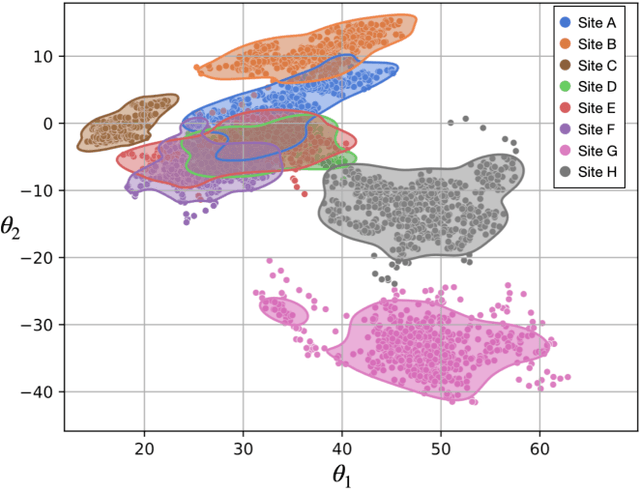
Abstract:Accuracy and consistency are two key factors in computer-assisted magnetic resonance (MR) image analysis. However, contrast variation from site to site caused by lack of standardization in MR acquisition impedes consistent measurements. In recent years, image harmonization approaches have been proposed to compensate for contrast variation in MR images. Current harmonization approaches either require cross-site traveling subjects for supervised training or heavily rely on site-specific harmonization models to encourage harmonization accuracy. These requirements potentially limit the application of current harmonization methods in large-scale multi-site studies. In this work, we propose an unsupervised MR harmonization framework, CALAMITI (Contrast Anatomy Learning and Analysis for MR Intensity Translation and Integration), based on information bottleneck theory. CALAMITI learns a disentangled latent space using a unified structure for multi-site harmonization without the need for traveling subjects. Our model is also able to adapt itself to harmonize MR images from a new site with fine tuning solely on images from the new site. Both qualitative and quantitative results show that the proposed method achieves superior performance compared with other unsupervised harmonization approaches.
A Structural Causal Model for MR Images of Multiple Sclerosis
Mar 05, 2021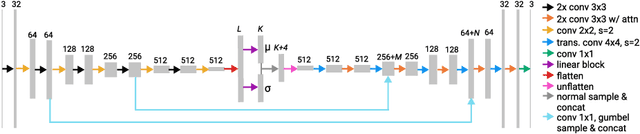

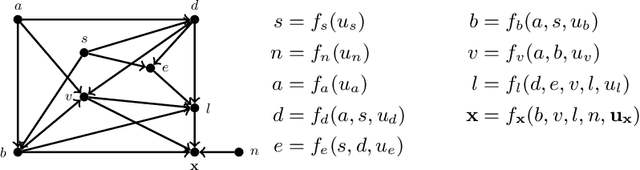
Abstract:Precision medicine involves answering counterfactual questions such as "Would this patient respond better to treatment A or treatment B?" These types of questions are causal in nature and require the tools of causal inference to be answered, e.g., with a structural causal model (SCM). In this work, we develop an SCM that models the interaction between demographic information, disease covariates, and magnetic resonance (MR) images of the brain for people with multiple sclerosis (MS). Inference in the SCM generates counterfactual images that show what an MR image of the brain would look like when demographic or disease covariates are changed. These images can be used for modeling disease progression or used for downstream image processing tasks where controlling for confounders is necessary.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge