Hemmings Wu
NeuroCLIP: A Multimodal Contrastive Learning Method for rTMS-treated Methamphetamine Addiction Analysis
Jul 27, 2025Abstract:Methamphetamine dependence poses a significant global health challenge, yet its assessment and the evaluation of treatments like repetitive transcranial magnetic stimulation (rTMS) frequently depend on subjective self-reports, which may introduce uncertainties. While objective neuroimaging modalities such as electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) offer alternatives, their individual limitations and the reliance on conventional, often hand-crafted, feature extraction can compromise the reliability of derived biomarkers. To overcome these limitations, we propose NeuroCLIP, a novel deep learning framework integrating simultaneously recorded EEG and fNIRS data through a progressive learning strategy. This approach offers a robust and trustworthy biomarker for methamphetamine addiction. Validation experiments show that NeuroCLIP significantly improves discriminative capabilities among the methamphetamine-dependent individuals and healthy controls compared to models using either EEG or only fNIRS alone. Furthermore, the proposed framework facilitates objective, brain-based evaluation of rTMS treatment efficacy, demonstrating measurable shifts in neural patterns towards healthy control profiles after treatment. Critically, we establish the trustworthiness of the multimodal data-driven biomarker by showing its strong correlation with psychometrically validated craving scores. These findings suggest that biomarker derived from EEG-fNIRS data via NeuroCLIP offers enhanced robustness and reliability over single-modality approaches, providing a valuable tool for addiction neuroscience research and potentially improving clinical assessments.
An End-to-End Deep Learning Approach for Epileptic Seizure Prediction
Aug 17, 2021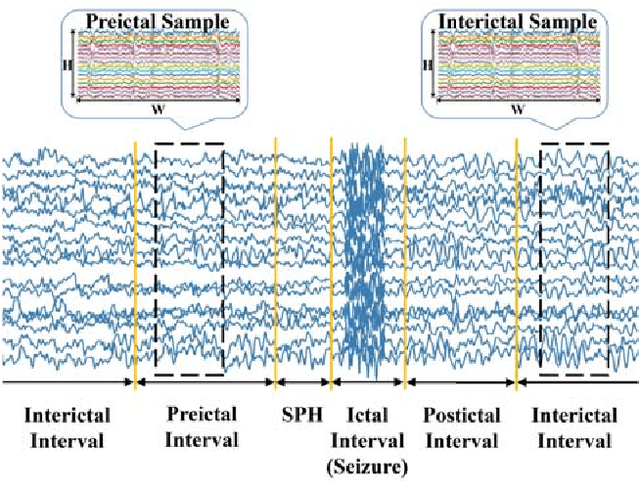
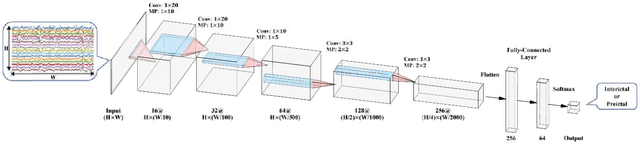
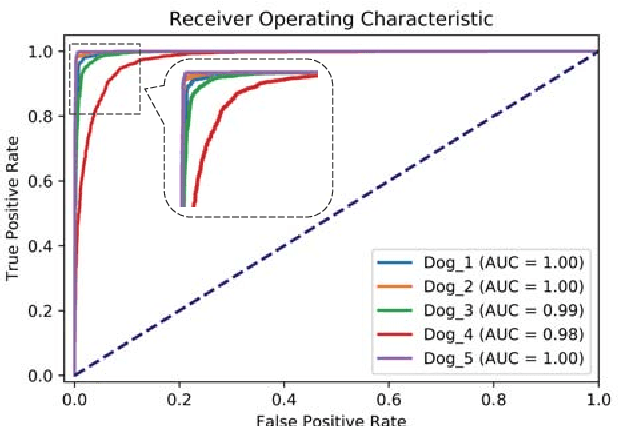
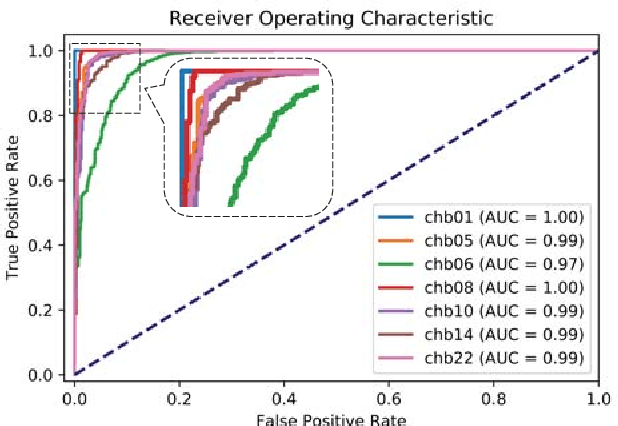
Abstract:An accurate seizure prediction system enables early warnings before seizure onset of epileptic patients. It is extremely important for drug-refractory patients. Conventional seizure prediction works usually rely on features extracted from Electroencephalography (EEG) recordings and classification algorithms such as regression or support vector machine (SVM) to locate the short time before seizure onset. However, such methods cannot achieve high-accuracy prediction due to information loss of the hand-crafted features and the limited classification ability of regression and SVM algorithms. We propose an end-to-end deep learning solution using a convolutional neural network (CNN) in this paper. One and two dimensional kernels are adopted in the early- and late-stage convolution and max-pooling layers, respectively. The proposed CNN model is evaluated on Kaggle intracranial and CHB-MIT scalp EEG datasets. Overall sensitivity, false prediction rate, and area under receiver operating characteristic curve reaches 93.5%, 0.063/h, 0.981 and 98.8%, 0.074/h, 0.988 on two datasets respectively. Comparison with state-of-the-art works indicates that the proposed model achieves exceeding prediction performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge